2. Guangzhou Aier Eye Hospital, Guangzhou 510062, China
2. 广州爱尔眼科医院,广东 广州 510062
The conventional chest tubes (CCT) have been in use for treatment of hemothorax and pneumothorax ever since the World War Ⅱ[1, 2]. Due to its thick diameter, hard texture and the liability of occlusion, the placement of CCT is a rather painful process and has a high postoperative malposition rate (up to 25.6%[3, 4]) and also a high incidence rate of complications (as high as 30%[5, 6]), some of which can even be fatal [7-9]. In addition, the removal of CCT can be even more painful. To reduce the pain and complications caused by CCT, small-bore pigtail catheters or central venous catheters were introduced, but these tubes are also liable to occlusion by blood clots and fibrous adhesions[10-12] and can be easily fractured when the lung expands, thus not suitable for hemothorax and empyema drainage[13, 14].
The minimally invasive uniportal video-assisted thoracic surgery (VATS) developed in recent years [15, 16] also uses CCT for postoperative drainage. But as the incision is commonly made at the fifth intercostal space along the midaxillary line, which is not a particularly good site for either fluid or air drainage, the CCT placed there tends to kink and has a poor drainage effect. In the case of infants, the use of CCT is even more problematic. Since currently there is no special chest tube for infants, surgeons have to use CCTs with a smaller diameter, but due to the small pleural space and the active movements of infants, such chest tubes may cause many complications.
To solve the problems with CCT, we designed a 14F minimal-invasive experimental chest tube (ECT) using polyurethane. In this study, we tested the performance of this new chest tube for blood drainage in the thoracic cavity of a rabbit model of hemothorax in comparison of with a 28F CCT, and evaluated the safety and potential application of this chest tube.
METHODS Design of the small-bore ECTThe small-bore ECT, with a diameter of 14-French (Fr) and a wall thickness of 1 mm, is made of polyurethane (PU), and is composed of 4 parts, i.e., the forepart, the intermediate part, the joint and the distal part (Fig. 1). The forepart has a circular design 2 cm in diameter with a tiny apex and a small bore that allows the passage of a guide wire of 0.035 inch. The forepart is 1.5 cm in length at the end after excluding the lateral aperture. The forepart and the intermediate part, which is 20 cm in length, form an angle of 90 degree (Fig. 1). The joint connects the intermediate part with the distal part, small at the proximal part and larger at the distal part. The distal part of the ECT is a 24 F tube made of silica gel with a spindle-shape balloon (20 mL) in the middle, which can be compressed after clamping the far end of the tube. The distal part connects the ECT with a chest drainage bottle (Fig. 2A).
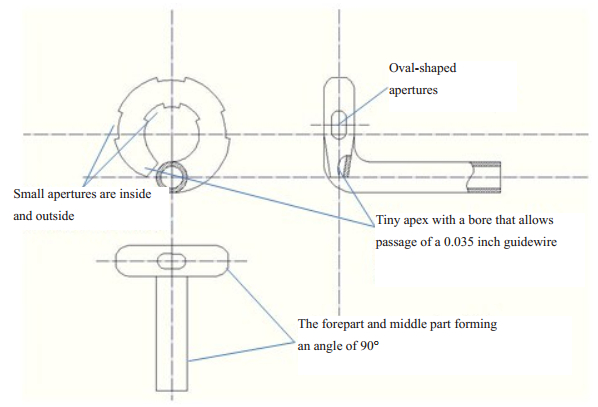
|
Figure 1 Design of the experimental chest drainage tube (ECT). |
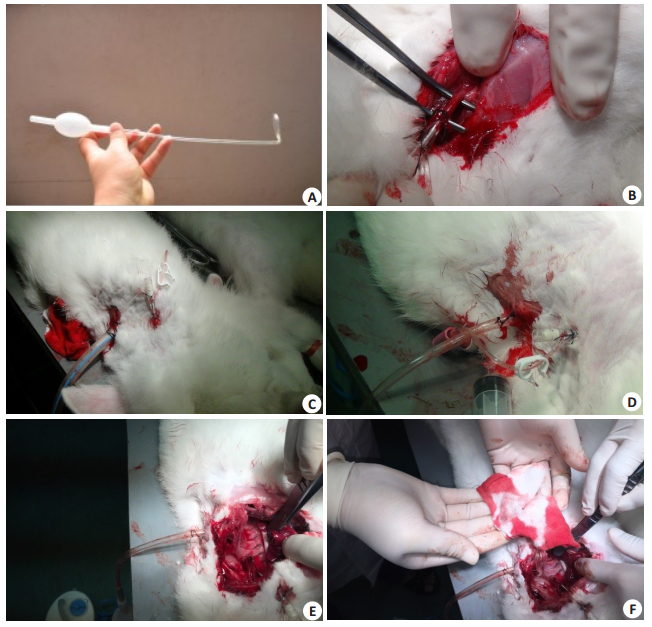
|
Figure 2 Images of the chest drainage tube experiment. A: Experimental chest tube (ECT); B: Exploration of the carotid artery in a blood provider rabbit (Group 3); C: 28 F conventional chest tube placed in a rabbit model and a detaining needle placed at the third intercostal space to inject blood into the pleural cavity; D: ECT placed in a rabbit model and a detaining needle placed at the third intercostal space to inject blood into the pleural cavity; E: No displacement of ECT was found after drainage with ECT for 120 min, almost without residue blood in the pleural cavity; F: After cleaning the pleural cavity with gauze, the residue blood was nearly zero. |
The study protocol was approved by the Ethics Committee of the Laboratory Animal Center at the Southern Medical University. A total of 30 male 5-month-old New Zealand white rabbits (body weight around 2.5 kg) provided by the Laboratory Animal Center of Southern Medical University were divided into experimental group (n=9), control group (n=6), and blood provider group (n=15). In the blood provider group, the blood-providing rabbits (BPR) were anesthetized with 1% sodium pentobarbital (30 mg/kg) via the auricular veins and fixed in a supine position on the platform with their left carotid arteries exposed (Fig. 2B). An intravenous catheter was inserted into the left carotid artery, around which the needle was fixed proximally and the carotid artery was ligated at the distal.
For establishment of models of hemothorax, the rabbits in the experiment group and control group (chest drainage rabbits, CDR) were anaesthetized and fixed in the same manner, and a 2-mm incision was made at the 3th intercostal place along the midclavicular line. An intravenous catheter (Fig. 2C, D) was inserted through the incision into the chest cavity, and after aspiration of the air entering the chest with a syringe, 20 mL of fresh blood collected from the carotid artery of the BPR was injected into the chest cavity. For chest tube placement, another incision (3 mm for ECT and 10 mm for CCT) was made at the 6th intercostal space along the midaxillary line. For ECT placement, after insertion of the tip into the thoracic cavity, the tube was rotated anticlockwise so that the forepart was placed closely against the chest wall (Fig. 2D). The CCT was placed as in routine practice, with the last lateral aperture about 1.5 cm away from the chest wall (Fig. 2C).
The time duration for the insertion and placement of chest tube was recorded in each operation. After chest tube placement, 20 mL fresh blood from BPR was infused into the pleural space of CDR every 20 min for 6 times. Both the ECT and CCT were compressed once every 20 min in the middle of the blood infusion twice, with the ECT clamped at the end of the distal part (distal to the balloon) and the CCT at the end of tube. The drainage volume was measured every 20 min prior to the next blood infusion. The total amount of drainage was recorded 20 min after the final blood infusion. Two hours later, the CDR was sacrificed by a lethal intravenous dose of 1% sodium pentobarbital (120 mg/kg), and thoracotomy was performed at the 4th intercostal space place for observing and weighing the residual blood and clots in the left pleura space (Fig. 2F).
The complications caused by chest tube drainage were also examined, including tube malposition, kinking, and injuries of the lungs, blood vessels, heart or abdominal organs.
Statistical analysesSPSS (SPSS Inc., Chicago, IL, USA) software version 13.0 was used for all statistical analyses. The continuous variables (body weight, drainage volume, and residual blood weight) are presented as Mean±SD. The differences between the two groups were assessed using two-sample t test. P < 0.05 was considered to indicate a statistically significant difference with a confidence interval [CI] of 95%.
RESULTSAs shown in Tab. 1, the mean body weight of the rabbits was similar between the experimental and control groups (P=0.485). The operation time for ECT placement was less than that of CCT placement (P=0.036). The drainage volume in the first and second 20 min was significantly greater in the experimental group than in the control group (P=0.02 and P=0.00, respectively), but in the 3rd, 4th, 5th and 6th 20 min, the drainage volumes were similar between the two groups (P > 0.05). The total volume of drainage was not significantly different between the two groups (P=0.596). The volume of residual blood and clots, however, was markedly lower in the experimental group than in the control group (P=0.011).
| Table 1 Drainage volume of the ECT and CCT in the rabbits during the experiment (Mean±SD) |
No complications other than abutment against lung were observed in the two groups. All the 6 CCTs were found to cause abutment pressure to the lung, while the ECT caused no such complications (P=0.00, Tab. 1).
DISCUSSIONIn this study, we tested the performance of the 14-Fr ECT for blood drainage in a rabbit model of hemothorax. The results showed that this new chest tube had a much better effect for blood drainage from the thoracic cavity than a 28-Fr CCT. This new ECT was also easier to deploy, less likely to fold or kink, and its placement can be less painful due to its smaller diameter. To our knowledge, this is the first report describing a superior drainage efficacy of a 14-Fr chest tube over a 28-Fr CCT.
Arakawa et al[17] developed a 20-Fr chest tube with an active cleaning system, whose drainage efficacy was better than that of a 32-Fr CTT; however, this chest tube was stiff and straight with a relatively large diameter, and still needs to be placed through an incision in the chest wall. In another report, Karasu et al[18] described comparable safety and efficacy of pigtail catheters (10-14 Fr) with those of the large-bore chest tube (20-28 Fr) in the treatment of secondary spontaneous pneumothorax.
Our new ECT may provide a good alternative for postoperative drainage following uni-portal VATS. As the forepart and middle part of the ECT forms a 90° angle, the forepart can be placed tightly to the pleural space to lower the risk of abutting such vital organs as the lung and the heart. As most of the uni-portal VATS are inserted at the fifth intercostal space[16, 19], which is not an ideal position for drainage of pleural fluid or air[20-22], the use of CCTs can be problematic as they easily get kinked or occluded when the lung re-expands [23-24]. In contrast, with lateral apertures at both the outside and the inside of the forepart circle, an ECT is not easily occluded, and its 2 cm-diameter circle at the forepart allows it to be placed with a considerable length in the chest, which further increase the lateral apertures and the opening area.
We recorded a significantly shorter operation time for ECT placement than for CCT placement, possibly due to the small diameter of the ECT. Its small diameter also allows it to be rotated anticlockwise after placement, which helps the operator to confirm whether it is placed at the right depth. We designed a small forepart of the ECT, because this tube was intended to be placed using the Seldinger method; however, as the rabbits have a quite small chest cavity with a chest volume similar to that of a neonate, using a very long guideware may cause injuries to the mediastinum. In further studies, we plan to verify the experiment results in larger animals such as pigs and dogs.
Tab. 1 shows that the ECT had a greater drainage efficiency than CCT in the first and second 20 min, but the total drainage volume was similar between the two groups, demonstrating an at least comparable drainage ability of ECT with that of the CCT. But the finding that the residual blood in the experimental group was much less than that in the control group indicates a greater drainage efficiency of the ECT than CCT. Because all the lateral apertures of the ECT are only 2 mm away from the chest wall, ECT can achieve more efficient drainage than CCT even when there are only a few pleural infusions. We actually observed nearly total blood drainage by ECT in two rabbits (Fig. 2E-F), which further demonstrate the great potential of ECT in the treatment of empyema. According to Carr et al [25], the formation of fluid compartment and pleural fibrosis is closely related to incomplete drainage of empyema, for the treatment of which Zahid et al [26] even favored thoracotomy over minimally invasive surgery. But open chest surgery has an inherent risk of injuries, and the use of ECT, with its excellent drainage ability, may totally replace open surgery.
In this study, we found that ECT did not cause any abutment injury to the lung, which appeared to be almost inevitable in all the 6 rabbits with CCT placement. In addition, as all chest tubes are liable to occlusion by blood clots, they need to be cleaned regularly during the drainage to maintain its patency. We designed a spindle-shaped balloon at the distal part of the ECT to allow compression of the tube for cleaning the distal part of tube, and this process can be quickly completed manually in 1 or 2 s.
Given the advantages of the ECT such as highly efficient drainage, good flexibility, good safety, as well as easy and less painful placement, we believe that this new chest tube can find potential clinical applications especially for closed thoracic drainage in infants or children and for blood drainage following single-port VATS.
Acknowledgement: We would like to thank Dr. JI Jie for assistance in the fabrication of the experimental drainage tube.| [1] | Monaghan SF, Swan KG. Tube thoracostomy: the struggle to the "standard of care"[J]. Ann Thorac Surg, 2008, 86(6): 2019-22. DOI: 10.1016/j.athoracsur.2008.08.006. |
| [2] | Betts RH, Lees WM. Military thoracic surgery in the forward area[J]. J Thorac Surg, 1946, 15(3): 44-63. |
| [3] | Dural K, Gulbahar G, Kocer B, et al. A novel and safe technique in closed tube thoracostomy[J]. J Cardiothorac Surg, 2010, 5(10): 21-5. |
| [4] | Chung MH, Hsiao CY, Nian NS, et al. The benefit of ultrasound in deciding between tube thoracostomy and observative management in hemothorax resulting from blunt chest trauma[J]. World J Surg, 2018. |
| [5] | Alrahbi R, Easton R, Bendinelli C, et al. Intercostal catheter insertion: are we really doing well?[J]. ANZ J Surg, 2012, 82(6): 392-4. DOI: 10.1111/ans.2012.82.issue-6. |
| [6] | Reinersman JM, Allen MS, Blackmon SH, et al. Analysis of patients discharged from the hospital with a chest tube in place[J]. Ann Thorac Surg, 2018, 105(4): 1038-43. DOI: 10.1016/j.athoracsur.2017.10.042. |
| [7] | Goltz JP, Gorski A, Bohler J, et al. Iatrogenic perforation of the left heart during placement of a chest drain[J]. Diagn Interv Radiol, 2011, 17(3): 229-31. |
| [8] | Haron H, Rashid NA, Dimon MZ, et al. Chest tube injury to left ventricle: complication or negligence?[J]. Ann Thorac Surg, 2010, 90(1): 308-9. |
| [9] | Rombola CA, Tomatis SB, Honguero MA, et al. Parapneumonic pleural effusion. Accidental insertion of a chest tube into right pulmonary artery[J]. Eur J Cardiothorac Surg, 2008, 34(4): 903-8. DOI: 10.1016/j.ejcts.2008.06.043. |
| [10] | Clark G, Licker M, Bertin D, et al. Small size new silastic drains: life-threatening hypovolemic shock after thoracic surgery associated with a non-functioning chest tube[J]. Eur J Cardiothorac Surg, 2007, 31(3): 566-8. DOI: 10.1016/j.ejcts.2006.12.010. |
| [11] | Cho S, Lee EB. Management of primary and secondary pneumothorax using a small-bore thoracic catheter[J]. Interact Cardiovasc Thorac Surg, 2010, 11(2): 146-9. DOI: 10.1510/icvts.2009.226589. |
| [12] | Cafarotti S, Dall'armi V, Cusumano GA, et al. Small-bore wire-guided chest drains: Safety, tolerability, and effectiveness in pneumothorax, malignant effusions, and pleural empyema[J]. J Thorac Cardiovasc Surg, 2011, 141(3): 683-7. DOI: 10.1016/j.jtcvs.2010.08.044. |
| [13] | Petel D, Li P, Emil S. Percutaneous pigtail catheter versus tube thoracostomy for pediatric empyema: A comparison of outcomes[J]. Surgery, 2013, 154(4): 655-60. DOI: 10.1016/j.surg.2013.04.032. |
| [14] | Eichhorn ME, Winter H, Preissler G, et al. Modern tailored therapy for pleural empyema[J]. Zentralbl Chir, 2011, 136(1): 34-41. DOI: 10.1055/s-0030-1262539. |
| [15] | Sihoe AD. The evolution of minimally invasive thoracic surgery: implications for the practice of uniportal thoracoscopic surgery[J]. J Thorac Dis, 2014, 6(6): S604-17. |
| [16] | Gonzalez-Rivas D. VATS lobectomy: surgical evolution from conventional VATS to uniportal approach[J]. Scien World J, 2012, 10(2): 780842-9. |
| [17] | Arakawa Y, Shiose A, Takaseya T, et al. Superior chest drainage with an active tube clearance system: evaluation of a downsized chest tube[J]. Ann Thorac Surg, 2011, 91(2): 580-3. |
| [18] | Tsai WK, Chen W, Lee JC, et al. Pigtail catheters vs large-bore chest tubes for management of secondary spontaneous pneumothoraces in adults[J]. Am J Emerg Med, 2006, 24(7): 795-800. DOI: 10.1016/j.ajem.2006.04.006. |
| [19] | Kutluk AC, Kocaturk CI, Akin H, et al. Which is the best minimal invasive approach for the treatment of spontaneous pneumothorax? Uniport, two, or three ports: a prospective randomized trail[J]. Thorac Cardiovasc Surg, 2018, 25(1): 24-33. |
| [20] | Karasu S, Tokat AO, Cetinkanat CG, et al. Benefits from apical chest tube drainage in pneumothorax[J]. Tohoku J Exp Med, 2012, 226(2): 145-50. DOI: 10.1620/tjem.226.145. |
| [21] | Wu MH, Wu HY. Pleural drainage using drainage bag for thoracoscopic lobectomy[J]. Asian Cardiovasc Thorac Ann, 2018, 26(3): 212-7. DOI: 10.1177/0218492318760876. |
| [22] | He J, Ma DJ, Li SQ. Uniportal video-assisted thoracoscopic right upper lobectomy and systemic mediastinal lymph nodes dissection[J]. J Thorac Dis, 2017, 9(6): 1644-7. DOI: 10.21037/jtd. |
| [23] | Chang SW, Ryu KM, Ryu JW. Delayed massive hemothorax requiring surgery after blunt thoracic trauma over a 5-year period: complicating rib fracture with sharp edge associated with diaphragm injury[J]. Clin Exp Emerg Med, 2018, 5(1): 60-5. DOI: 10.15441/ceem.16.190. |
| [24] | Mendes MA, China PN, Ribeiro C, et al. Conventional versus pigtail chest tube - are they similar for treatment of malignant pleural effusions[J]. Support Care Cancer, 2018, 25(19): 1-4. |
| [25] | Carr JA, Fales C, Shaikh IA, et al. Computed tomographic modeling before and after treatment for posttraumatic empyema: early decortication is superior to catheter drainage[J]. Ann Thorac Surg, 2011, 91(6): 1723-8. DOI: 10.1016/j.athoracsur.2011.02.046. |
| [26] | Zahid I, Routledge T, Bille A, et al. What is the best treatment of postpneumonectomy empyema?[J]. Interact Cardiovasc Thorac Surg, 2011, 12(2): 260-4. DOI: 10.1510/icvts.2010.254706. |
 2018, Vol. 38
2018, Vol. 38

