Xenotransplantation mouse models provide important means to carry out in vivo studies of the pathogenesis of human diseases and investigate the mechanisms that regulate the growth and differentiation of human stem cells [1]. The engraftment of human cells or integrating human genes in immunodeficient mouse models allows the development of humanized mice for biomedical research. Among the various strains of immunodeficient mice, nude mice are the most widely used [2] for their lack of T cells, but the presence of B cells and natural killer (NK) cells causes difficulties in stable and persistent human cell reconstitution in the host. The mutation of Prkdc (protein kinase, DNA activated, catalytic polypeptide) gene or recombination-activating gene 1 (Rag1)/Rag2 genes impairs the repair mechanisms of double-strand DNA breaks and prevents V(D)J recombination of B cell receptor and T cell receptor recombination, thus leading to the absence of lymphocytes and severe combined immunodeficiency (SCID) [3, 4]. The limitations that impede human cell engraftment in SCID and Rag1/Rag2-deficient mice [5, 6] include the activity of host NK cells, which cause rejection of the engrafted human cells. In NOD mouse strain, the signal regulatory protein-α (SIRP-α) expressed on macrophages is mutated and binds to human CD47 with a high affinity, resulting in the inhibition of xenotransplant destruction by host macrophages [7, 8]. The development of NOD/LtSz-scid mice[9] and NOD/Shi-scid mice with a relatively low activity of NK cells and macrophage- mediated rejection promotes the success of human cell engraftment.
A major breakthrough in elimination of NK cells in SCID mice was the discovery that the disruption of interleukin-2 receptor (IL-2R) γ-chain (IL2Rγ) abolished NK cell development. IL2Rγ is shared by the receptors for IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21, and is required for signaling through these receptors. In addition to the absence of NK cells, the disruption of IL2Rγ also leads to defective B and T cell development and function [10]. Therefore, among the various strains of immunodeficient mice, NOD/SCID/IL2Rγ-/- (NSG) mice [11, 12] can be most efficiently engrafted with human cells and allow reconstitution with human hematopoietic stem cells (HSCs) and peripheral blood mononuclear cells (PBMCs) to generate mouse models with humanized immune system. These humanized mouse models can be widely used to study human immune responses to cancer, infection, and transplantation immunology[4, 13].
Recent development of type Ⅱ clustered regularly interspaced short palindromic repeat (CRISPR)- associated (Cas9) system has enabled highly efficient gene editing in various species [14-18]. Cas9 can be directed by a single guide RNA (gRNA) to generate DNA double-strand breaks (DSBs) at a specific genomic site, which can be repaired either imprecisely by nonhomologous end-joining (NHEJ) or precisely by homology-directed repair (HDR) [19]. In the present study, we disrupted Prkdc and IL2Rγ genes in Balb/c mice using CRISPR/Cas9 system, and intercrossed the genetically modified mice with NOD mice to generate a new strain of NSG mice (referred to herein as cNSG mice). The cNSG mice were characterized by total deletion of B, T, and NK cells, and can be genotyped easily with PCR to detect the mutations.
METHODS Mouse strainsAll the animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC). Balb/c (L2015037) and NOD/SCID mice were purchased from Charles River Co. (Beijing, China). Nude mice were purchased from Laboratory Animal Center of Southern Medical University (Guangzhou, China). All the mice were housed and bred in specific pathogen-free (SPF) grade cages and provided with autoclaved food and water.
Plasmid constructionThe gRNAs were designed using the online software (http://www.genome-engineering.org). The complementary oligos for each target sequence were heated at 95 ℃ for 5 min, annealed, and the short double-strand DNA fragments were ligated into pT7-BbsI-gRNA vector. The sequence of gRNAs is listed in Tab. 1.
| Table 1 Sequences of the single guide RNAs (gRNAs) of the target genes |
In vitro transcription of gRNAs was performed with MEGAshortscript Kit (Ambion, AM 1354) according to the manufacturer's recommendation, and the transcribed gRNAs were recovered with MEGAclear Kit (Ambion, AM1908). The Cas9 mRNA expression vector was linearized with Sph I (NEB) for use as the template for in vitro transcription with the mMESSAGE mMACHINE T3 Transcription Kit (Ambion, AM 1348).
Microinjection of one-cell embryosTo obtain the oocytes, female Balb/c mice (4-6 weeks old) were superovulated by intraperitoneal injection of 10 IU pregnant mare serum gonadotropin (PMSG, SANSHENG) and 10 IU human chorionic gonadotropin (HCG, SANSHEN) at a two-day interval. After the injection of HCG, the female Balb/c mice were mated with male Balb/c mice (8-10 weeks old), and 16-18 h later, the fertilized embryos were collected from the oviducts. The embryos were injected with a mixture of gRNA (50 ng/μL) and Cas9 mRNA (100 ng/μL) and transferred to the oviduct of 0.5-day pseudopregnant female mice.
DNA extraction and genotypingThe genomic DNA was extracted from the ear tissue of the mice using SDS lysis buffer. The genomic DNA was subjected to PCR amplification to identify the mutations. The Sirpa mutation was confirmed by sequencing the PCR product, and the primers used are listed in Tab. 2.
| Table 2 Sequence of the primers used for genotyping the mutant mice |
Single cell suspensions were prepared from samples of mouse peripheral blood, bone marrow, spleen and thymus, and were stained for 30 min with the following antibodies (purchased from BD Biosciences): anti-mCD45- APC-Cy7 (clone 30-F11), anti-mCD3e-PE-Cy7 (clone 145-2C11), anti-mCD19-PerCP-Cy5.5 (clone 1D3), anti-mCD4-APC (clone RM4-5), anti-mCD8-FITC (clone 53-6.7), and anti-mCD49b-PE (clone DX5). The dead cells were excluded with DAPI staining. The positively stained cells were analyzed by flow cytometer (BD LSRFortessa) and the data were analyzed using FlowJo software. We used the Graph Pad to analyze the data by One-way ANOVA.
Cell cultureHuman lung adenocarcinoma A549 cell line and nasopharyngeal carcinoma 5-8F cell line were cultured in DMEM and RPMI-1640 medium supplemented with 10% fetal bovine serum, and were passaged when reaching 80% confluence.
Tumor cell transplantationTo compare the tumor engraftment efficiency of cNSG and nude mice, we transplanted human lung adenocarcinoma A549 cells (5 × 106 per mouse) and human nasopharyngeal carcinoma 5-8F cells (5×106 per mouse) subcutaneously in the mice (aged 4-6 weeks, body weight 19-22 g, n=5 for each strain). The mice were monitored daily and the tumor size was measured with a caliper. The tumor volume (TV) was calculated using the formula: TV=A·B2/2, where A is the length (mm) and B the width (mm) of the tumor. Seven days after the transplantation, the mice were sacrificed and the tumors were weighed.
Statistical analysisThe differences in engraftment rates among different mouse strains were analyzed using two-way ANOVA. Student's t-test was used to determine the statistical significance of the tumor weight. A P value less than 0.05 was considered to indicate a statistically significant difference. All the statistical analyses were performed using Prism software, version 6.0 (GraphPad, Inc, San Diego, CA, USA).
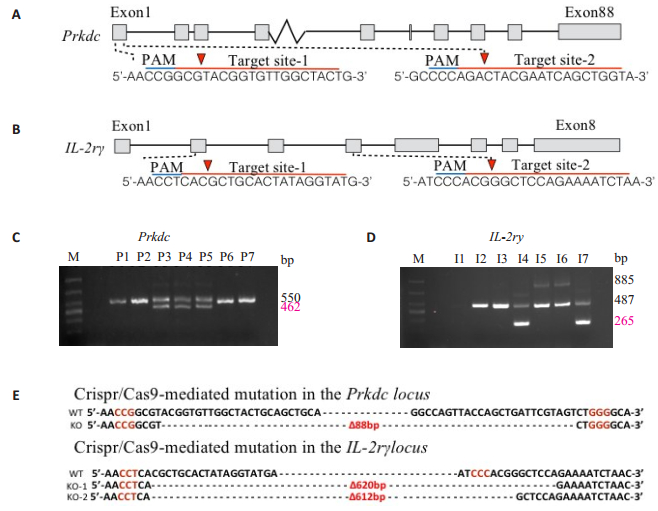
RESULTS Generation of Prdkc-/- and IL2Rγ-/- miceThe strategy to generate knockout mice with CRISPR/ Cas9 technology is illustrated in Fig. 1. We designed two gRNAs targeting exon 1 of mouse Prkdc gene (Fig. 2A), and two gRNAs to delete the genomic DNA that spanned exons 2-4 of IL2Rγ gene (Fig. 2B). The mixture of Cas9 mRNA and gRNAs was injected into the cytoplasm of one-cell embryos, which were transplanted in pseudo-pregnant female mice. The offspring mice were genotyped by PCR to identify the targeted mutations. The mutant allele of Prkdc gene gave rise to a 462-bp PCR product, and the wide-type (WT) allele to a 550-bp PCR product. The mutant allele of IL2Rγ gene yielded a 265-bp PCR product and the WT allele yielded 885-bp and 487-bp PCR products. Sequencing analysis of the PCR products revealed disruption of both of the gene loci in the genome of the mice (Fig. 2C).
|
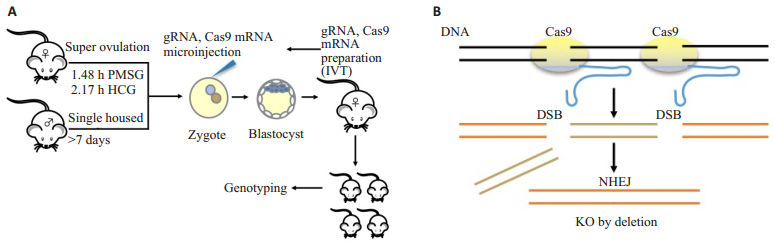
Figure 1 Strategy to generate knockout mouse using CRISPR/Cas9 technology. A: Schematic illustration of pronuclear microinjection. The fertilized eggs were collected and microinjected with gRNA/Cas9 mRNA into the cytoplasm. The injected zygotes were incubated for 5-6 h before transfer into pseudopregnant surrogate mothers. B: Mechanism of CRISPR/Cas9-mediated gene knockout. Single guide RNA (gRNA) consisting of 20-nt guide sequence (blue) and a scaffold (red) was recognized by the protospacer-adjacent motif (PAM) of Cas9. The sequence 5'-NGG adjacent motif was paired with the DNA target site to induce DNA double strand breaks (DSBs) ~3 bp upstream of the PAM (red triangle). The repair via non-homologous end joining (NHEJ) results in random insertional or deletional mutations at the site of DNA breaks. |
|
Figure 2 Targeted disruption of Prkdc gene and IL2Rγ gene in mice via CRISPR/Cas9 nuclease. A: gRNAs were designed to delete the exon 1 of mouse Prkdc gene locus. B: gRNAs were designed to delete exons 2-4 of the mouse IL2Rγ gene locus. C, D: PCR analysis confirming the knockout of Prkdc gene and IL2Rγ gene in the founder mice. E: The deleted sequences of Prkdc and IL2Rγ genes revealed by sequencing of the PCR products of the targeted allele. The wild-type (WT) sequence is shown on the top. Deletion is indicated as ∆n bp and PAM NGGs highlighted in red. |
On average, 20-40 embryos were collected from each mouse. Thirty injected embryos were transferred into the oviduct on each side of 10 pseudopregnant female mice, and 8 of them gave birth to a total of 40 pups. The Prdkc and IL2Rγ mutant mice were intercrossed to generate double heterozygous mutant mice, and 10 male double heterozygous mice were mated with 30 NOD mice (8-10 weeks old) to obtain 15 NOD/Prdkc +/-IL2Rγ+/- mice. These mice were backcrossed 5 generations to obtain at least 25 NOD/Prdkc-/-IL2Rγ-/- mice (cNSG mice).
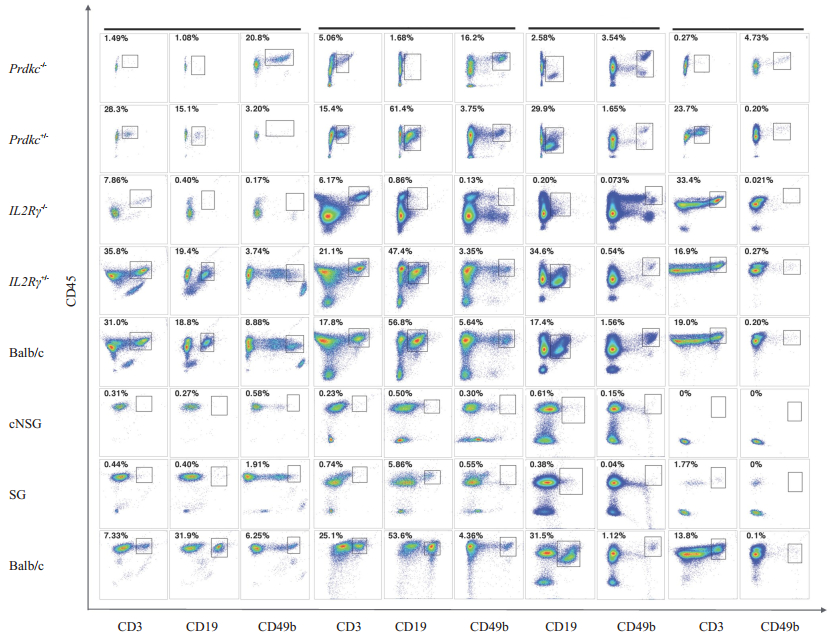
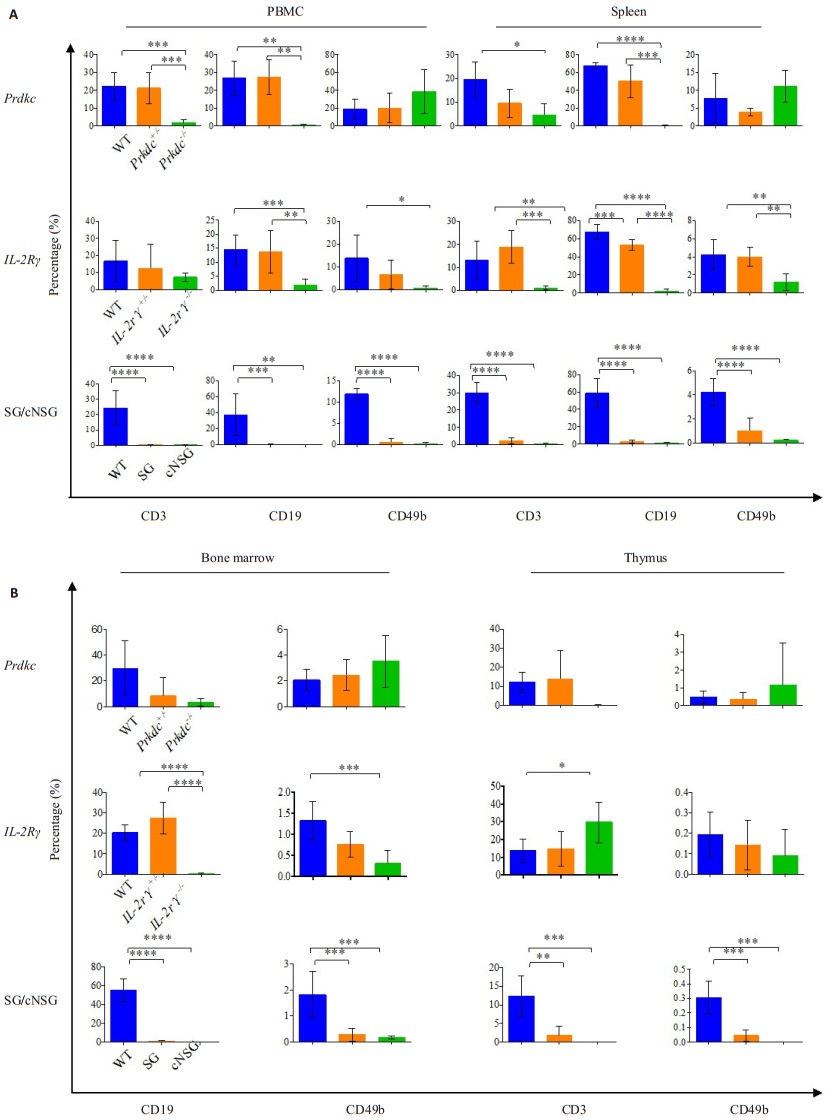
Immunodeficiency phenotypes of the mutant miceTo determine the effects of loss of the Prkdc gene on the immune system, we analyzed the lymphocytes in the PBMCs, spleen, bone marrow and thymus of 5- to 8-week-old mice. As expected, the development of both B cells and T cells were blocked in Prdkc-/- mice, while the NK cells were slightly elevated (Fig. 3, 4). B cell development appeared to be completely blocked, but residual T cells were detected in the Prdkc-/- mice. In IL2Rγ-/- mice, the disruption of IL2Rγ gene led to the total absence of NK cells and mature B cells and defective development of T cells (Fig. 3, 4).
|
Figure 3 Flow cytometric analysis of different mutant mice. Representative data from flow cytometric analysis of the immune cells in the PBMCs, spleen, bone marrow and thymus of WT, Prdkc+/-, Prdkc-/-, IL2Rγ+/-, IL2Rγ-/-, SG and cNSG mice. The percentages of the gated cell population in total CD45+ leukocytes are indicated. |
|
Figure 4 Statistical analysis of the percentage of T, B and NK cells in the PBMCs and spleen (A) and in the bone marrow and thymus (B) of different mutant mice. Statistical data are shown of WT, Prdkc+/-, Prdkc-/-, IL2Rγ+/-, IL2Rγ-/-, SG and NSG mice. The number of mice analyzed: Balb/c mice (n=10), Prkdc mice (n=4), IL2Rγ mice (n=6), SG mice (n=5), cNSG mice (n=5). *P≤0.05, **P≤0.01, ***P≤0.001, ****P≤0.00001. |
Analysis of the Prdkc-/-IL2Rγ-/- (SG) mice and cNSG mice showed that mature T cells, B cells and NK cells were all absent in the PBMCs, spleen and thymus, demonstrating that cNSG mice had equivalent phenotype of immunodeficiency to the existing NSG mice (Fig. 3, 4).
To test whether cNSG mice could be more efficiently engrafted with human tumor cells than the most commonly used immunodeficient nude mice, human tumor cells were injected subcutaneously into the flanks of cNSG and nude mice. Tumors grew much faster in cNSG mice than in nude mice (Fig. 5A, C). In addition, the tumors formed by human cancer cells were significantly larger and heavier than those in nude mice (Fig. 5B, D, E, F). We therefore conclude that human tumor cells can be more efficiently grafted in cNSG mice than in nude mice.
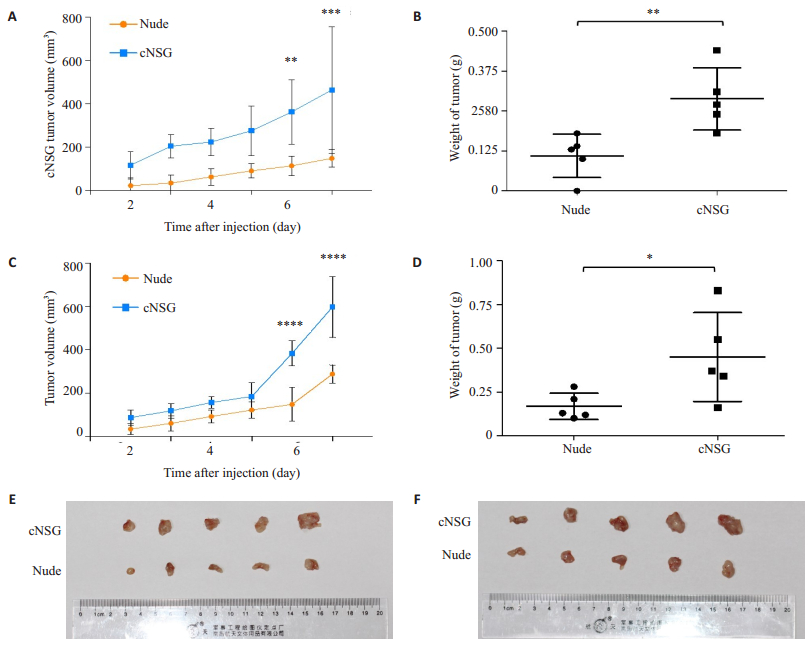
|
Figure 5 More effective engraftment of human tumor cells in cNSG mice than in nude mice. Growth curves of tumors formed by A549 cells (A) and 5-8F cells (C) indicate that human tumor cells grew much faster in cNSG mice than in nude mice. The weight of xenograft tumors formed by A549 cells (B) and 5-8F cells (D) in nude and cNSG mice was measured at day 7 after transplantation, and the images of tumors formed by A549 cells (E) and 5-8F cells (F) in nude and cNSG mice. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. |
The homozygous mutation in Prkdc gene results in SCID with multiple defects in adaptive and innate immunity. We show here that Prdkc mutant mice have slightly elevated levels of endogenous NK cells, which is consistent with the findings in previous studies [3]. Treatment with anti-NK cell antibodies enhances the engraftment rates of human cells in SCID mice, indicating that NK cells play a crucial role in the rejection of xenografts[20, 21]. While a targeted mutation in the β2-microglobulin gene leads to severe defects in NK cell activity, mice defective in both Prdkc and β 2-microglobulin gene exhibit a markedly shortened life span due to the accelerated development of thymic lymphomas[22, 23]. The development of thymic lymphomas in NOD/SCID mice is dependent on cytokine signaling mediated through IL2Rγ [11]. Therefore, IL2Rγ deficiency not only abolishes NK cell development but also prevents the development of thymic lymphomas in NSG mice, making them ideal mouse models for xenotransplantation studies.
SIRPα protein expressed on mouse macrophages, due to its poor interaction with human CD47, plays a key role in macrophage-mediated rejection of xenografts. NOD mutation in SIRPα protein improves the interaction between mouse SIRPα and human CD47, and thus prevents macrophage-mediated rejection of human cells by the host [7, 8, 24]. Therefore, SCID/IL2Rγ-/- mice or RAG-/-/IL2Rγ-/- mice will not be suitable xenotransplantation models for human cells[25-27].
Using the CRISPR/Cas9 system, we generated a new strain of NSG mice with NOD mutation and total deletion of mature B, T and NK cells. The cNSG mice exhibit the same levels of immunodeficiency as the existing NSG mice maintained by Jackson's Laboratory in United States. The SCID mutation in the Jackson's NSG strain is a spontaneous mutation that is difficult to be genotyped. In contrast, SCID mutation in cNSG mice is a deletional mutation of the Prdkc gene that can be easily identified by PCR. Considering that the Jackson's NSG mice are currently not available in China, cNSG mice will provide an important tool for biomedical research in China.
| [1] | Yahata T, Ando K, Nakamura Y, et al. Functional human T lymphocyte development from cord blood CD34+ cells in nonobese diabetic/Shi-scid, IL-2 receptor gamma null mice[J]. J Immunol, 2002, 169(1): 204-9. DOI: 10.4049/jimmunol.169.1.204. |
| [2] | Flanagan SP. 'Nude', a new hairless gene with pleiotropic effects in the mouse[J]. Genet Res, 1966, 8(3): 295-309. DOI: 10.1017/S0016672300010168. |
| [3] | Bosma GC, Custer RP, Bosma MJ. A severe combined immunodeficiency mutation in the mouse[J]. Nature, 1983, 301(5900): 527-30. DOI: 10.1038/301527a0. |
| [4] | Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research[J]. Nat Rev Immunol, 2007, 7(2): 118-30. DOI: 10.1038/nri2017. |
| [5] | Mombaerts P, Iacomini J, Johnson RS, et al. RAG-1-deficient mice have no mature B and T lymphocytes[J]. Cell, 1992, 68(5): 869-77. DOI: 10.1016/0092-8674(92)90030-G. |
| [6] | Shinkai Y, Rathbun G, Lam KP, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement[J]. Cell, 1992, 68(5): 855-67. DOI: 10.1016/0092-8674(92)90029-C. |
| [7] | Takenaka K, Prasolava TK, Wang JC, et al. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells[J]. Nat Immunol, 2007, 8(12): 1313-23. DOI: 10.1038/ni1527. |
| [8] | Yamauchi T, Takenaka K, Urata S, et al. Polymorphic Sirpa is the genetic determinant for NOD-based mouse lines to achieve efficient human cell engraftment[J]. Blood, 2013, 121(8): 1316-25. DOI: 10.1182/blood-2012-06-440354. |
| [9] | Shultz LD, Schweitzer PA, Christianson SW, et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice[J]. J Immunol, 1995, 154(1): 180-91. |
| [10] | Cao X, Shores EW, Hu-Li J, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain[J]. Immunity, 1995, 2(3): 223-38. DOI: 10.1016/1074-7613(95)90047-0. |
| [11] | Ishikawa F, Yasukawa M, Lyons B, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor chain (null) mice[J]. Blood, 2005, 106(5): 1565-73. DOI: 10.1182/blood-2005-02-0516. |
| [12] | Shultz LD, Lyons BL, Burzenski LM, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells[J]. J Immunol, 2005, 174(10): 6477-89. DOI: 10.4049/jimmunol.174.10.6477. |
| [13] | Shultz LD, Brehm MA, Garcia-Martinez JV, et al. Humanized mice for immune system investigation: progress, promise and challenges[J]. Nat Rev Immunol, 2012, 12(11): 786-98. DOI: 10.1038/nri3311. |
| [14] | Carbery ID, Ji D, Harrington A, et al. Targeted genome modification in mice using zinc-finger nucleases[J]. Genetics, 2010, 186(2): 451-9. DOI: 10.1534/genetics.110.117002. |
| [15] | Shao Y, Guan Y, Wang L, et al. CRISPR/Cas-mediated genome editing in the rat via direct injection of one-cell embryos[J]. Nat Protoc, 2014, 9(10): 2493-512. DOI: 10.1038/nprot.2014.171. |
| [16] | Sommer D, Peters A, Wirtz T, et al. Efficient genome engineering by targeted homologous recombination in mouse embryos using transcription activator-like effector nucleases[J]. Nat Commun, 2014, 5: 3045. DOI: 10.1038/ncomms4045. |
| [17] | Sung YH, Baek IJ, Kim DH, et al. Knockout mice created by TALEN-mediated gene targeting[J]. Nat Biotechnol, 2013, 31(1): 23-4. DOI: 10.1038/nbt.2477. |
| [18] | Wang H, Yang H, Shivalila CS, et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering[J]. Cell, 2013, 153(4): 910-8. DOI: 10.1016/j.cell.2013.04.025. |
| [19] | Ran FA, Hsu PD, Wright J, et al. Genome engineering using the CRISPR-Cas9 system[J]. Nat Protoc, 2013, 8(11): 2281-308. DOI: 10.1038/nprot.2013.143. |
| [20] | Koyanagi Y, Tanaka Y, Tanaka R, et al. High levels of viremia in hu-PBL-NOD-scid mice with HIV-1 infection[J]. Leukemia, 1997, 11(Suppl): 3109-112. |
| [21] | Ueda T, Tsuji K, Yoshino H, et al. Expansion of human NOD/ SCID-repopulating cells by stem cell factor, Flk2/Flt3 ligand, thrombopoietin, IL-6, and soluble IL-6 receptor[J]. J Clin Invest, 2000, 105(7): 1013-21. DOI: 10.1172/JCI8583. |
| [22] | Christianson SW, Greiner DL, Hesselton RA, et al. Enhanced human CD4 + T cell engraftment in beta2-microglobulin-deficient NOD-scid mice[J]. J Immunol, 1997, 158(8): 3578-86. |
| [23] | Feuring-Buske M, Gerhard B, Cashman J, et al. Improved engraftment of human acute myeloid leukemia progenitor cells in beta 2-microglobulin-deficient NOD/SCID mice and in NOD/SCID mice transgenic for human growth factors[J]. Leukemia, 2003, 17(4): 760-3. DOI: 10.1038/sj.leu.2402882. |
| [24] | Leijon K, Hammarstrom B, Holmberg D. Non-obese diabetic (NOD) mice display enhanced immune responses and prolonged survival of lymphoid cells[J]. Int Immunol, 1994, 6(2): 339-45. DOI: 10.1093/intimm/6.2.339. |
| [25] | Li F, Cowley DO, Banner D, et al. Efficient genetic manipulation of the NOD-Rag1-/-IL2RgammaC-null mouse by combining in vitro fertilization and CRISPR/Cas9 technology[J]. Sci Rep, 2014, 45290. |
| [26] | Wei X, Lai Y, Li B, et al. CRISPR/Cas9-mediated deletion of Foxn1 in NOD/SCID/IL2rg(-/-) mice results in severe immunodeficiency[J]. Sci Rep, 2017, 7(1): 7720. DOI: 10.1038/s41598-017-08337-8. |
| [27] | Zhao Y, Liu PJ, Bai B, et al. Construction of severe combined immunodeficiency mice based on CRSIPR/Cas9 technology[J]. Acta Lab Anim Sci Sin, 2016, 4(24): 339-43. |
 2018, Vol.
2018, Vol. 

