2. 中山大学附属第一医院东院 儿科,广东 广州 510700;
3. 中山大学附属第一医院内分泌科,广东 广州 510080
2. Department of Pediatrics, Huangpu Division of First Affiliated Hospital of Sun Yat-sen University, Guangzhou 510700, China;
3. Department of Endocrinology, First Affiliated Hospital of Sun Yat-sen University, Guangzhou 510080, China
糖尿病是一种以高血糖为特征的代谢性疾病,而大血管并发症是其致死、致残最主要的原因[1]。高血糖引起血管内皮细胞(VECs)功能失调是血管病的始动因子[2-4]。探讨高血糖引起VEC损伤的病理生理机制对防治糖尿病血管病的发生极其重要。糖原合酶激酶-3(GSK-3)[5]及内质网应激(ERS)在糖尿病心血管并发症中的作用日益受到重视[6]。
GSK-3是一种丝氨酸/苏氨酸激酶,被认为是多种细胞事件和信号通路的调节者,包括GSK-3α和GSK-3β两个成员。与大部分的蛋白激酶不同,GSK-3在磷酸化状态时是不活动的。GSK- 3α结构中的Ser21或GSK-3β中的Ser9去磷酸化导致它们被激活并对各种刺激产生反应[7-8]。糖尿病肾病大鼠肾组织的GSK-3β活性增强,GSK-3β抑制剂可抑制GSK-3β活性,减轻肾组织的病理改变及尿蛋白量[9]。高糖引起H9C2细胞凋亡及GSK-3β活性增强,小干扰RNA沉默GSK-3β后能保护心肌细胞对抗高糖引起的损伤[5]。在糖尿病大鼠,GSK- 3β抑制剂通过调控Nrf2/TrxR2信号通路保护肾脏对抗缺血再灌注引起的损伤[10]。有关GSK-3β在高糖损伤血管内皮细胞的作用及其机制尚未见相关研究。
内质网应激(ERS)是指某些因素使ER生理功能发生紊乱的一种亚细胞器病理状态。高浓度的葡萄糖能激活内皮细胞,但长期高血糖则导致持续的ERS[11]。适当程度的ERS对维持细胞的稳定是有利的,而长期的ERS则能引起细胞凋亡及发生许多疾病[12]。ERS在T2DM的VECs损伤中起着重要的作用[13-15]。高糖能引起人脐静脉内皮细胞(HUVECs)凋亡、ERS及内皮素-1生成增多;Mir-149-5P能通过抑制ERS(降低GRP78、CHOP表达)及增加抗凋亡蛋白(Bcl-2)表达等机制保护HUVECs对抗高糖引起的损伤[6]。在胰岛瘤细胞模型,ERS引起的凋亡与增加GSK-3β活性有关[16]。在脂多糖(LPS)诱发的肝损伤中,GSK-3β与促进ERS引起的凋亡有关[17]。因此,我们推测在高糖引起的VECs损伤中,可能存在GSK-3β与ERS相互作用,而且这种相互作用可能是高糖损伤VECs的一个重要的病理生理机制。
本研究拟建立高糖处理的HUVECs损伤模型,探讨GSK-3β与ERS是否存在相互作用;GSK-3β和ERS在高糖损伤血管内皮细胞中的多种作用,为深入阐明高糖对血管内皮细胞的损伤机制提供实验依据。
1 材料和方法 1.1 材料和试剂抗GSK- 3β、抗磷酸化(p)-GSK-3β(Ser9)、抗GRP78、抗CHOP和抗cleaved caspase-3抗体购自Cell Signaling;双氯荧光素(DCFH-DA)、罗丹明123(Rh 123)、Hoechst 33258染料、氯化锂(LiCl,GSK-3β的抑制剂)、4-苯基丁酸(4-PBA,ERS的抑制剂)购自SigmaAldrich;特级胎牛血清(FBS)购自Gibco BRL;细胞计数试剂盒8(CCK-8)由Dojindo Lab(Japan)提供;DMEM培养基由Hyclone供应。HUVECs由中山大学实验动物中心细胞库供应。
1.2 细胞培养及实验分组在含5% CO2的37 ℃恒温培养箱中、采用含10% FBS的DMEM培养HUVECs,当细胞生长至约80%的融合状态时可进行实验。实验分为6组:(1)Con组:DMEM培养基作用HUVECs 24 h;(2)高糖(HG)组:40 mmol/L葡萄糖作用HUVECs 24 h;(3)LiCl+ HG组:10 mmol/L LiCl预处理HUVECs 60 min,再予40 mmol/L葡萄糖作用24 h;(4)4-PBA+HG组:20 μmol/L 4-PBA预处理HUVECs 60 min,再予40 mmol/L葡萄糖作用24 h;(5)LiCl组:10 mmol/L LiCl预处理HUVECs 60 min,再予DMEM作用24 h;(6)4-PBA组:20 μmol/L 4-PBA预处理HUVECs 60 min,再予DMEM作用24 h。
1.3 GSK-3β、GRP78、CHOP和cleaved caspase-3蛋白水平测定HUVECs在60 mm培养皿中生长至约80%融合度时,给予不同的处理后加入细胞裂解液作用30 min后,高速离心10 min后取上清液进行蛋白定量(BCA法)。等量蛋白经十二烷基硫酸钠聚丙烯酰胺凝胶(SDSPAGE)电泳分离后,转移至PVDF膜上,5%脱脂奶粉常温孵育1 h后加入Ⅰ抗稀释液[包括抗GSK-3β、抗pGSK-3β(Ser9)、抗cleaved caspase-3、抗GRP78、抗CHOP和GAPDH,浓度均为1:1000],4 ℃作用过夜后加入Ⅱ抗稀释液(浓度为1:3000)孵育1.5 h。ECL法使PVDF膜显色,暗室中曝光到X线片上,凝胶成像扫描系统分析结果。实验重复5次。
1.4 细胞存活率测定在96孔板中培养HUVECs,当细胞生长至约80%的融合度时,按各分组处理后,吸去细胞培养液,PBS液冲洗3次,加入CCK-8溶液10 μL/孔,37 ℃恒温培养箱内孵育2.5 h,酶标仪上读取A 450 nm;细胞存活率按照以下公式计算:细胞存活率(%)=处理组A /对照组A× 100%。实验重复5次。
1.5 细胞凋亡检测HUVECs在24孔板中生长至约80%的融合度时,按照分组给予不同处理后,4%的多聚甲醛固定10 min,加入用Hoechst 33258染料稀释液,37 ℃温箱中作用30 min,荧光显微镜下可观察到:正常的细胞呈弥散均匀的低密度荧光,凋亡细胞则表现为细胞核呈现浓缩致密的固缩形态或颗粒荧光,随机照片记录5个高倍镜视野(放大200倍,以下同)。应用Image J 1.47i软件计算平均荧光强度(MFI),对每组数据进行统计分析。实验重复5次。
1.6 细胞内ROS水平测定HUVECs在24孔板中生长至融合度约80%时,经不同处理后,加入DCFH-DA染液后于37 ℃温箱中作用30 min,荧光显微镜(TE-2000 Nikon)下随机照片记录5个高倍镜视野,应用Image J 1.47i计算出绿色荧光的MFI(数值大小能间接反映ROS水平的高低),并对每组数据进行统计分析。实验重复5次。
1.7 线粒体膜电位(MMP)水平测定HUVECs在24孔板中生长至约80%的融合度时,按照分组给予不同处理后,加入Rh 123染液,37 ℃温箱中作用30 min,荧光显微镜下随机照片记录5个高倍镜视野,应用图像分析软件计算绿色荧光的MFI(数值大小可反映MMP的高低),并对每组数据进行统计分析。实验重复5次。
1.8 统计学处理利用SPSS 19.0软件进行数据分析,结果以均数±标准差表示,单因素方差分析用于多个样本均数间的比较,结果有统计学意义再采用SNK-q检验进行均数间的两两比较,P < 0.05为差异有统计学意义。
2 结果 2.1 高糖激活人脐静脉内皮细胞GSK-3β40 mmol/L葡萄糖(HG)作用人脐静脉内皮细胞(HUVECs)1~24 h对磷酸化(p)-GSK-3β的影响(图 1A、B)。当HG作用HUVECs 3 h可使p-GSK-3β的表达水平明显下降(反映GSK-3β被激活),与Con组比较,差异具有统计学意义(P < 0.05);随着作用时间的延长(6、9、12、24 h),呈显著的时间-剂量依赖关系,与Con组比较,差异具有统计学意义(P < 0.01)。在上述作用时间段,HG对HUVECs的GSK-3β表达水平无明显的影响。
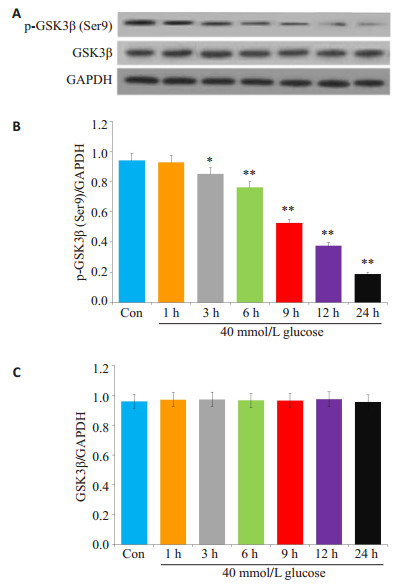
|
图 1 高糖对HUVECs的GSK-3β的激活作用 Figure 1 Effect of high glucose (HG, 40 mmol/L) exposure for 24 h on activation of glycogen synthase kinase- 3β (GSK- 3β) in human umbilical vein endothelial cells (HUVECs). A: Expression levels of p-GSK-3β and GSK- 3βsemi- quantified by Western blot analysis; B, C: Densitometric analysis of p- GSK- 3β and GSK- 3β expression levels. *P < 0.05, **P < 0.01 vs control group (n=5) |
HG作用HUVECs 1 h可明显地增加GRP78的表达水平,与Con组比较,差异具有统计学意义(P < 0.05,图 2A、B)。随着作用时间的延长(3、6、9、12、24 h),HG对GRP78表达水平的促进作用呈显著的时间-剂量依赖关系,与Con组比较,差异具有统计学意义(P < 0.01);HG作用HUVECs 3 h开始引起CHOP蛋白表达显著增多,随着作用时间的延长,这种作用呈显著的时间-剂量依赖关系(P < 0.01,图 2A、C)。
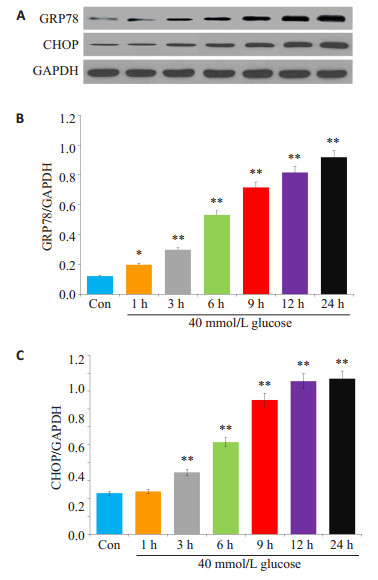
|
图 2 高糖引起HUVECs的ERS Figure 2 HG (40 mmol/L) exposure for 24 h induces endoplasmic reticulum stress (ERS) in HUVECs. A: Expression levels of GRP78 and CHOP semi-quantified by Western blot analysis; B, C: Densitometric analysis of GRP78 and CHOP expression levels. *P < 0.05, **P < 0.01 vs control group (n=5) |
当HG作用HUVECs 24 h可明显地诱发ERS,GRP78和CHOP的表达水平明显升高(P < 0.01)。在HG产生作用前,应用10 mmol/L LiCl(GSK-3β抑制剂)预处理HUVECs 60 min,HG对GRP78和CHOP表达的促进作用明显减弱,与HG组比较,差异具有统计学意义(P < 0.01,图 3)。单独应用10 mmol/LLiCl处理HUVECs 60 min,HG对GRP78和CHOP表达水平影响不明显。
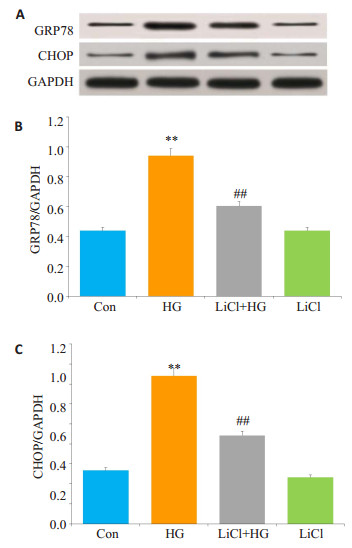
|
图 3 活化的GSK-3β介导HG引起的HUVECs ERS Figure 3 Activated GSK-3β mediates HG-induced ERS in HUVECs. HUVECs were pre-treated with 10 mmol/L LiCl for 60 min before exposure to 40 mmol/L glucose (HG) for 24 h. A: Expression levels of GRP78 and CHOP semi-quantified by Western blot analysis; B, C: Densitometric analysis of GRP78 and CHOP expression levels. **P < 0.01 vs control group; ##P < 0.01 vs HG-treated cels (n=5). HG: High glucose |
HG作用HUVECs 24 h可激活GSK-3β,p-GSK-3β表达水平明显降低,与Con组比较,差异具有统计学意义(P < 0.01,图 4A、B)。在HG产生作用前,应用20 μmol/L 4-PBA(ERS抑制剂)预处理HUVECs 60 min,p-GSK- 3β表达水平升高,与单独HG组比较,差异具有统计学意义(P < 0.01)。单独20 μmol/L 4-PBA处理HUVECs 60 min,p-GSK-3β表达水平的影响不显著。
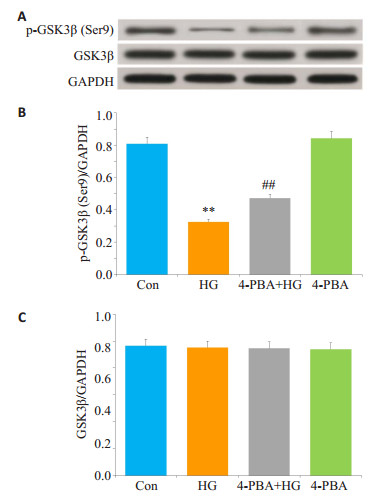
|
图 4 ERS参与高糖对HUVECs GSK-3β的激活作用 Figure 4 ERS is involved in HG-induced GSK-3β activation in HUVECs. HUVECs were pre-treated with 20 μmol/L 4-PBA for 60 min before exposure to 40 mmol/L glucose (HG) for 24 h. A: Expression levels of p- GSK- 3β and GSK-3βsemi-quantified by Western blot analysis. B, C: Densitometric analysis of p-GSK-3β and GSK-3β expression levels. **P < 0.01 vs control group; ##P < 0.01 vs HG-treated group (n=5). HG: High glucose |
HG处理HUVECs 24 h可引起明显的细胞毒性作用,细胞存活率明显降低,与Con组比较,差异具有统计学意义(P < 0.01,图 5)。在HG处理前,应用10 mmol/L LiCl或20 μmol/L 4-PBA预处理HUVECs 60 min,可分别对抗HG引起的细胞毒性,使细胞存活率升高,与单独HG组比较,差异具有统计学意义(P < 0.01)。单独用10 mmol/L LiCl或20 μmol/L 4-PBA处理HUVECs 60 min,对细胞存活率影响不明显。
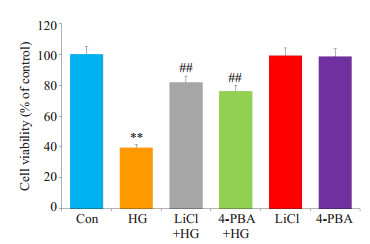
|
图 5 GSK-3β和ERS参与高糖引起的HUVECs存活率降低 Figure 5 GSK-3β and ERS are implicated in HG-induced decrease in HUVEC viability. HUVECs were treated with 40 mmol/L glucose for 24 h with or without pre- treatment with 10 mmol/L LiCl or 20 μmol/L 4-PBA for 60 min. Cell viability was detected using the Cell counting Kit-8 (CCK-8) assay. **P < 0.01 vs control group; ##P < 0.01 vs HG-treated group (n=5). HG: High glucose |
HG作用HUVECs 24 h可引起凋亡细胞数量显著增加,与Con组比较,差异具有统计学意义(P < 0.01,图 6A、B)。在HG作用前,应用10 mmol/L LiCl或20 μmol/L 4-PBA预处理HUVECs 60 min,均能明显地减小凋亡细胞数量,与单独HG组比较,差异均具有统计学意义(P < 0.01)。用10 mmol/L LiCl或20 μmol/L 4-PBA处理HUVECs 60 min,对凋亡细胞数量无明显的影响。HG处理HUVECs 24 h引起cleaved caspase-3[为凋亡效应器)表达水平明显升高,与Con组比较,差异具有统计学意义(P < 0.01,图 6C~F)]。在HG处理前,应用10 mmol/L LiCl或20 μmmol/L 4-PBA预处理60 min,均能明显地减少cleaved caspase-3的表达水平,与HG处理组比较,差异均具有统计学意义(P < 0.01)。用10 m mmol/L LiCl或20 μmmol/L 4-PBA处理HUVECs 60 min,对cleaved caspase-3的表达水平无明显的影响。
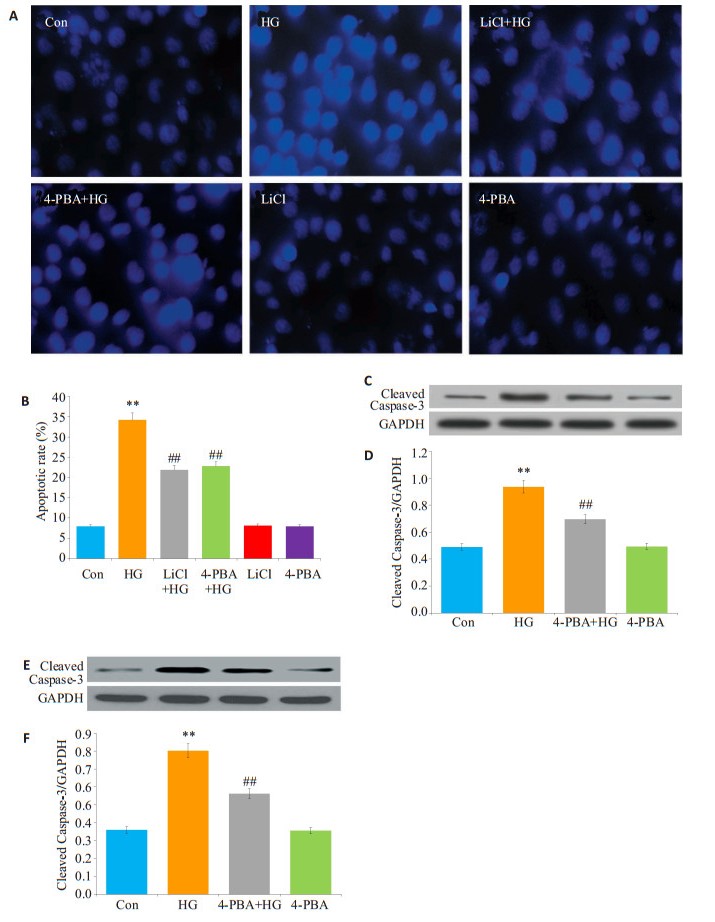
|
图 6 GSK-3β和ERS参与高糖引起的HUVECs凋亡 Figure 6 GSK-3β and ERS participate in HG-induced apoptosis in HUVECs. HUVECs were treated with 40 mmol/L glucose for 24 h with or without pre-treatment with 10 mmol/L LiCl or 20 μmol/L 4-PBA for 60 min. A: Detection of cell apoptosis using Hoechst 33258 nuclear staining followed by fluorescence imaging; B: Percentage of apoptotic cells in different groups analyzed using Image J 1.47i software; C, E: Expression levels of cleaved caspase-3 semi-quantified by Western blot analysis; D, F: Densitometric analysis of cleaved caspase-3 expression levels. **P < 0.01 vs the control group; ##P < 0.01 vs HG-treated group (n=5). HG: High glucose |
应用DCFH-DA染色法检测细胞内活性氧(ROS)水平。HG处理HUVECs 24 h,胞内DCFH-DA的MFI明显升高,与Con组比较,差异具有统计学意义(P < 0.01,图 7A、B)。在HG处理前,应用10 mmol/L LiCl或20 μmol/L 4-PBA预处理HUVECs 60 min,均能降低胞内DCFH-DA的MFI,与HG组分别比较,差异均具有统计学意义(P < 0.01)。10 mmol/L LiCl或20 μmol/L 4- PBA处理HUVECs 60 min,对胞内ROS水平表达无明显影响。
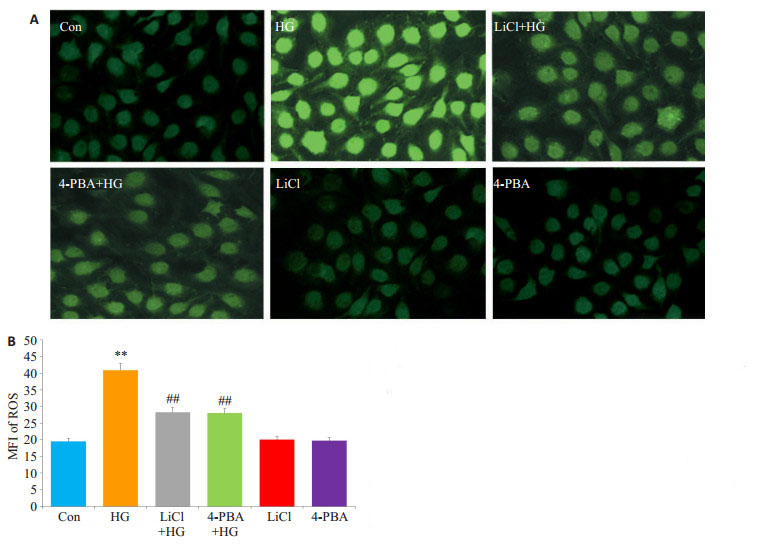
|
图 7 GSK-3β和ERS参与高糖引起的HUVECs的氧化应激 Figure 7 GSK-3β and ERS are involved in HG- induced oxidative stress in HUVECs. HUVECs were treated with 40 mmol/Lglucose for 24 h with or without pre-treatment with 10 mmol/L LiCl or 20 μmol/L 4-PBA for 60 min. A: Intracellular ROS generation measured by 2'7-dichlorodihydrofluoresein diacetate (DCFHDA) staining followed by photofluorography; B: Quantitative analysis of the mean fluorescence intensity (MFI) using Image J 1.47i software. **P < 0.01 vs control group; ##P < 0.01 vs HG-treated group (n=5). HG: High glucose |
HG处理HUVECs 24 h引起线粒体损伤,线粒体膜电位(MMP)丢失,即Rh123荧光染料的MFI降低,与Con组相比,差异具有统计学意义(P < 0.01,图 8A、B)。在HG产生作用前,应用10 mmol/L LiCl或20 μmol/L4-PBA预处理HUVECs 60 min,均能拮抗HG引起的MMP丢失,与HG处理组分别比较,差异具有统计学意义(P < 0.01)。单独应用10 mmol/LLiCl或20 μmol/L4-PBA处理HUVECs 60 min,对MMP水平表达无明显影响。
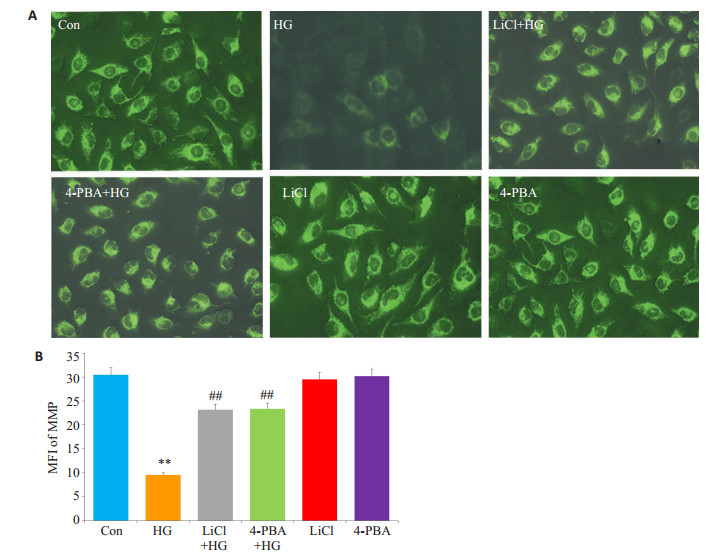
|
图 8 GSK-3β和ERS参与高糖引起的人脐静脉内皮细胞MMP丢失 Figure 8 GSK-3β and ERS contribute to HG-induced dissipation of mitochondrial membrane potential in HUVECs. HUVECs were treated with 40 mmol/L glucose for 24 h with or without pre-treatment with 10 mmol/L LiCl or 20 μmol/L 4-PBA for 60 min. A: MMP detected using the fluorescent dye Rh123; B: Quantitative analysis of the mean fluorescence intensity (MFI) using Image J 1.47i software. **P < 0.01 vs control group; ##P < 0.01 vs HG-treated group (n=5). HG: High glucose |
高血糖是引起糖尿病心血管并发症的最重要因素[17-19]。高血糖可通过引起细胞凋亡、细胞毒性、炎症、氧化应激、损伤线粒体等作用引起血管内皮细胞功能障碍[20-22]。内皮素-1[6]、氧化应激[21]、瘦素/瘦素受体通路[21]、JAK/STAT通路[22]及沉默信息调节子1[23]等参与高糖对血管内皮细胞的损伤。GSK-3(特别是GSK-3β)[5]及ERS[6]在糖尿病心血管并发症中的作用受到学者们的关注。
GSK-3β是导致胰岛素缺失和胰岛素抵抗的一个重要因素[24]。高糖引起H9C2心肌细胞GSK-3β活性增强,抑制GSK-3β能减轻高糖引起心肌细胞凋亡[5]。在高糖处理的HUVECs模型,GSK-3β活性增强、细胞凋亡增多、线粒体膜通透性丢失及细胞色素C释放增多[25]。该研究没有应用GSK-3β抑制剂或小干扰RNA沉默GSK-3β等方法进一步证实GSK-3β在高糖损伤HUVECs中的作用[25]。本研究观察到高糖能引起HUVECs多种损伤,表现为细胞存活率降低、凋亡细胞数量增多、ROS生成增多及MMP丢失;另外,高糖能增加HUVECs GSK-3β活性,这与Liu等人的报道相一致[25]。本研究应用GSK-3β抑制剂后,高糖引起的上述内皮细胞损伤明显减轻,提示GSK-3β参与高糖引起的细胞毒性、细胞凋亡、氧化应激及线粒体损伤,进一步证实了GSK-3β在高糖损伤血管内皮细胞中的作用[25]。
抑制ERS能减轻2型糖尿病心肌梗塞[26]。高糖能引起HUVECs发生ERS和细胞凋亡[27]。本研究证实糖能在HUVECs诱发ERS,表现为GRP78和CHOP蛋白表达增多,这与高糖作为应激刺激激发ERS有关。4- PBA能保护血管内皮细胞对抗高糖引起的损伤,表现为细胞存活率升高,凋亡细胞数量、ROS生成及MMP丢失减少,提示ERS参与高糖引起的血管内皮细胞多种损伤[28-30],这为把ERS作为一个靶点来防治高糖引起的血管并发症提供了实验依据。
综上所述,在高糖处理的HUVECs,应用LiCl能抑制高糖引起的ERS,4-PBA能抑制高糖对GSK-3β的激活作用。这证明GSK-3β与ERS在高糖损伤HUVECs过程中,存在着相互作用,即激活的GSK-3β能加强ERS,ERS也能促进GSK-3β激活,这可能是高糖损伤血管内皮细胞的作用机制,为防治糖尿病血管并发症提供了一个新思路。
| [1] |
Koizumi K, Wang G, Park L. Endothelial dysfunction and Amyloid- β-Induced neurovascular alterations[J].
Cell Mol Neurobiol, 2016, 36(2): 155-65.
DOI: 10.1007/s10571-015-0256-9. |
| [2] |
Favero G, Paganelli C, Buffoli B, et al. Endothelium and its alterations in cardiovascular diseases: Life style intervention[J].
Biomed Res Int, 2014(2): 801896.
|
| [3] |
Collino M, Aragno M, Castiglia S, et al. Insulin reduces cerebral ischemia/reperfusion injury in the hippocampus of diabetic rats: a role for glycogen synthase kinase-3beta[J].
Diabetes, 2009, 58(1): 235-42.
DOI: 10.2337/db08-0691. |
| [4] |
Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030[J].
Diabetes Res Clin Pract, 2010, 87(1): 4-14.
DOI: 10.1016/j.diabres.2009.10.007. |
| [5] |
Ren XM, Zuo GF, Wu W, et al. Atorvastatin alleviates experimental diabetic cardiomyopathy by regulating the GSK-3β-PP2Ac-NF-κB signaling axis[J].
PLoS One, 2016, 11(11): e0166740.
DOI: 10.1371/journal.pone.0166740. |
| [6] |
Yuan J, Chen M, Xu Q, et al. Effect of the diabetic environment on the expression of MiRNAs in endothelial cells: Mir-149-5p restoration ameliorates the high Glucose-Induced expression of TNF-α and ER stress markers[J].
Cell Physiol Biochem, 2017, 43(1): 120-35.
DOI: 10.1159/000480330. |
| [7] |
Vickers C, Hales P, Kaushik V, et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase[J].
J Biol Chem, 2002, 277(17): 14838-43.
DOI: 10.1074/jbc.M200581200. |
| [8] |
Oyadomari S, Araki E, Mori M. Endoplasmic reticulum stressmediated apoptosis in pancreatic beta-cells[J].
Apoptosis, 2002, 7(4): 335-45.
DOI: 10.1023/A:1016175429877. |
| [9] |
周谊霞, 李龙, 于燕妮, 等. 糖原合酶激酶-3β抑制剂对糖尿病肾病大鼠Wnt和核因子-κB信号通路的影响[J].
中华病理学杂志, 2015, 44(11): 783-7.
DOI: 10.3760/cma.j.issn.0529-5807.2015.11.005. |
| [10] |
Hu B, Wu Y, Liu J, et al. GSK-3beta inhibitor induces expression of Nrf2/TrxR2 signaling pathway to protect against renal ischemia/ reperfusion injury in diabetic rats[J].
Kidney Blood Press Res, 2016, 41(6): 937-46.
DOI: 10.1159/000452598. |
| [11] |
Witte I, Horke S. Assessment of endoplasmic reticulum stress and the unfolded protein response in endothelial cells[J].
Methods Enzymol, 2011, 489(489): 127-46.
|
| [12] |
Yoshida H. ER stress and diseases[J].
FEBS J, 2007, 274(3): 630-58.
DOI: 10.1111/ejb.2007.274.issue-3. |
| [13] |
Muoio DM, Newgard CB. Insulin resistance takes a trip through the ER[J].
Science, 2004, 306(5695): 425-6.
DOI: 10.1126/science.1104680. |
| [14] |
Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes[J].
Science, 2004, 306(5695): 457-61.
DOI: 10.1126/science.1103160. |
| [15] |
Basha B, Samuel SM, Triggle CR, et al. Endothelial dysfunction in diabetes mellitus: possible involvement of endoplasmic reticulum stress[J].
Exp Diabetes Res, 2012(2): 481840.
|
| [16] |
Srinivasan S, Ohsugi M, Liu Z, et al. Endoplasmic reticulum stressinduced apoptosis is partly mediated by reduced insulin signaling through phosphatidylinositol 3-kinase/Akt and increased glycogen synthase kinase-3beta in mouse insulinoma cells[J].
Diabetes, 2005, 54(4): 968-75.
DOI: 10.2337/diabetes.54.4.968. |
| [17] |
Chen L, Ren F, Zhang H, et al. Inhibition of glycogen synthase kinase 3β ameliorates D-GalN/LPS-induced liver injury by reducing endoplasmic reticulum stress-triggered apoptosis[J].
PLoS One, 2012, 7(9): e45202.
DOI: 10.1371/journal.pone.0045202. |
| [18] |
Brownlee M. Biochemistry and molecular cell biology of diabetic complications[J].
Nature, 2001, 414(6865): 813-20.
DOI: 10.1038/414813a. |
| [19] |
Xu WM, Wu W, Chen JF, et al. Exogenous Hydrogen sulfide protects H9c2 cardiac cells against high glucose-induced injury by inhibiting the activities of the p38 MAPK and ERK1/2 pathways[J].
Int J Mol Med, 2013, 32(4): 917-25.
DOI: 10.3892/ijmm.2013.1462. |
| [20] |
Sena CM, Pereira AM, Seica R. Endothelial dysfunction-A major mediator of diabetic vascular disease[J].
Biochim Biophys Acta, 2013, 1832(12): 2216-31.
DOI: 10.1016/j.bbadis.2013.08.006. |
| [21] |
吴冬波, 陈景福, 许庆, 等. 外源性硫化氢通过调控瘦素/瘦素受体通路抑制高糖诱导的人脐静脉内皮细胞损伤[J].
南方医科大学学报, 2016, 36(8): 1055-61.
|
| [22] |
林佳琼, 陈景福, 廖静秋, 等. 外源性硫化氢通过抑制JAK/STAT通路对抗高糖引起的人脐静脉内皮细胞损伤[J].
中国病理生理杂志, 2016, 32(7): 1161-6.
|
| [23] |
田飒, 彭学军, 何宇明, 等. 硫化氢通过调控沉默信息调节子1抑制高糖诱导的人脐静脉内皮细胞氧化应激损伤[J].
中国医药导报, 2014, 11(29): 19-23.
|
| [24] |
Zhang Y, Huang NQ, Yan F, et al. Diabetes mellitus and Alzheimer's disease: GSK-3β as a potential Link[J].
Behav Brain Res, 2018, 339(339): 57-65.
|
| [25] |
Liu H, Peng H, Chen S, et al. S1PR2 antagonist protects endothelial cells against high glucose-induced mitochondrial apoptosis through the Akt/GSK-3β signaling pathway[J].
Biochem Biophys Res Commun, 2017, 490(3): 1119-24.
DOI: 10.1016/j.bbrc.2017.06.189. |
| [26] |
Barr LA, Shimizu Y, Lambert JP, et al. Hydrogen sulfide attenuates high fat diet- induced cardiac dysfunction via the suppression of endoplasmic reticulum stress[J].
Nitric Oxide, 2015, 46(46): 145-56.
|
| [27] |
Zhang LM, Jiang CX, Liu DW. Hydrogen sulfide attenuates neuronal injury induced by vascular dementia via inhibiting apoptosis in rats[J].
Neurochem Res, 2009, 34(11): 1984-92.
DOI: 10.1007/s11064-009-0006-9. |
| [28] |
Pandey A, Saxena K, Verma M, et al. Correlative study between neuron- specific enolase and blood sugar level in ischemic stroke patients[J].
J Neurosci Rural Pract, 2011, 2(1): 50-4.
DOI: 10.4103/0976-3147.80099. |
| [29] |
Elgebaly MM, Ogbi S, Li W, et al. Neurovascular injury in acute hyperglycemia and diabetes: A comparative analysis in experimental stroke[J].
Transl Stroke Res, 2011, 2(3): 391-8.
DOI: 10.1007/s12975-011-0083-3. |
| [30] |
Bellolio MF, Gilmore RM, Stead LG. Insulin for glycaemic control in acute ischaemic stroke[J].
Cochrane Database Syst Rev, 2011, 7(9): CD005346.
|
 2018, Vol. 38
2018, Vol. 38

