根据最新的国内肿瘤调查数据, 肺癌仍是国内发病率最高的恶性肿瘤[1]。虽然目前肺癌治疗的手段增多、临床疗效明显提高, 但因其高转移性导致病人生存期和预后仍不理想[2-3]。因此, 进一步探讨肺癌转移的机制, 是目前肺癌研究的重点。脂肪酸转运蛋白4 (FABP4)是细胞内脂肪酸代谢的重要蛋白[4-5], 新近的研究发现其还可促进宫颈癌[6]、肝癌[7]等细胞增殖、转移。研究还发现, 其表达水平和肺癌的预后相关[8], 但其与肺癌细胞转移的关系目前鲜有报道。miRNA是调控细胞内蛋白表达的重要因子[9-10], miRNA是否通过调控FABP4表达影响肺癌的转移, 目前尚未见报道。因此, 本研究皆指在探讨FABP4对肺癌细胞转移能力的影响并研究靶向FABP4的miRNA。
1 材料和方法 1.1 材料FABP4兔来源多克隆一抗(CST, 美国), β-actin鼠来源单克隆一抗(CST, 美国), 抗兔或鼠HRP标记二抗(中杉金桥, 北京), 胰蛋白酶(吉诺公司, 中国), 高糖型DMEM、胎牛血清(Gibco, 美国), Transwell小室(Corning, 美国), 新霉素和嘌呤霉素(Sigma, 美国), Trizol试剂(Invitrogen, 美国); SYBR Green Real-time PCR Master Mix (Roche, 美国)。
1.2 细胞培养肺癌细胞株L9981、A549、PC13、NL9980、H661和95C由本实验室保存细胞。用含10%胎牛血清的DMEM培养基, 在5% CO2的37 ℃孵箱中培养。
1.3 shRNA质粒转染shRNA质粒由上海吉玛生物公司合成。质粒系列参考Wang等[11]的报道, shFABP4-1 sense, 5'-CACGAGA GAUUUAUGAGAdTdT-3'和antisense, 5'-UCUCUCA UAAACUCUCGUGdTdT-3'; shFABP4-2 sense, 5'-GC AUGGCCAAACCUAACAUTdT-3'和antisense, 5'-AU GUUAGGUUUGGCCAUGCdTdT-3'; shFABP4-3 sense, 5'-GGGAACCUUUCCACACUAUTT-3'和antisense, 5'-AUAGUGUGGAAAGGUUCCCTT-3'; NC sense, 5'-UUCUCCGAACGUGUCACGUTT-3'和antisense, 5'-ACGUGACACGUUCGGAGAATT-3。' NL9980细胞转染按lip-2000的说明书进行。转染48 h, 与新霉素0.15 μg/mL筛选, 直至荧光显微镜下95%以上的细胞有荧光, Western blot检测蛋白水平。
1.4 慢病毒转染过表达FABP4蛋白的慢病毒由上海和元生物公司合成。按慢病毒滴度和细胞比1:25进行转染A549细胞, 48 h后给予嘌呤霉素(500 ng/mL)筛选, 直至荧光显微镜下95%以上的细胞有荧光, Western blot检测蛋白水平。
1.5 ELISA检测以含1% BSA的PBS溶液为封闭液, 350 μL/孔, 室温封闭1 h。封闭结束后, 弃去封闭液, 洗板, 加入待测样品(细胞培养基上清液, 制作标准曲线以标准Purified Rat IgG代替样品)100 μL, 室温25±2 ℃孵育2 h。一抗孵育结束后, 洗板, 每孔加Anti-Rat IgG-HRP Antibody 100 μL, 室温孵育2 h。酶标二抗孵育结束后, 洗板, 每孔加入TMB底物溶液100 μL, 室温避光显色30 min后, 每孔加入2 mol/L H2SO4 50 μL终止反应。用酶标仪处检测A450 nm值。绘制标准曲线, 通过标准曲线计算样品浓度。
1.6 Western blot检测提取全细胞蛋白, 按体积的1/4加入5× loading buffer, 沸水煮10 min蛋白变性后-20 ℃保存。SDSPAGE胶分离蛋白(80 V 0.5 h后换100 V 1 h), 转膜(冰浴, 90 V 1.5 h)。封闭(5%脱脂奶粉, 室温, 2 h), 5% BSA稀释FABP4、β-actin一抗(1:1000)4 ℃过夜孵育, TBST 5 min/次× 3次。二抗(1:5000)常温1 h, TBST 5 min/次×3次洗膜后ECL显影。
1.7 Transwell检测50 mg/L Matrigel 1:5稀释液, 50 μL包被Transwell小室底部膜的上室面, 4 ℃风干。使用前用上室加入100 μL无血清培养基DMEM 37 ℃湿化30 min。细胞消化后用无血清培养基DMEM制成细胞悬液, 调整细胞密度为5×104/mL。吸取200 μL细胞悬液加入Transwell小室上室, 下室加入含10%血清的DMEM 600 μL。18 h后取出小室, 去除培养基, 4%多聚甲醛固定10 min后, 结晶紫染色2 h, 显微镜下拍照。
1.8 荧光素酶报告系统由广州赛哲生物公司设计和合成带荧光酶的质粒, 方法参考Zhang等[12]文献报道。转染NL9980细胞。参考Hidaka等[13]的方法, 在24孔板中加入PLB裂解液100 μL, 常温下裂解30 min后收集于一干净的EP管中。将样品加入白色96孔板中, 加入Luciferase Assay BufferⅡ后酶标仪检测荧光值Luc1, 再加入Stop & Glo® Buffer检测荧光值Luc2。Luc1/Luc2即为检测值。
1.9 Q-PCR检测miRNA胰酶消化收集L9981、A549、PC13、NL9980、H661和95C细胞, Trizol重悬后-80 ℃保存。冷冻后样品交付广州锐博生物公司, 其负责miR-129、miR-186、miR-203、miR-204、miR-361和miR-539的检测。反馈数据按2-△△法进行计算。
1.10 miRNA转染miRNA模拟物和抑制物(inhibitor)购至广州锐博生物公司, 按lip-2000的说明书进行转染。
1.11 统计学方法应用SPSS 13.0统计学软件, 利用Levene方法进行方差齐性检验, 确定方差齐性后, 采用单因素方差分析法, 整体比较组间差异有统计学意义后进一步作多重比较, 多重比较采用LSD法。以P < 0.05为差异有统计学意义。
2 结果 2.1 不同转移能力的肺癌细胞FABP4的分泌和表达水平选择不同转移能力的肺癌细胞株L9981、A549、PC13、NL9980、H661和95C, 检测FABP4表达情况。通过ELISA检测培养基上清中FABP4的表达, 结果显示低转移能力的L9981、A549、PC13分泌FABP4的水平较高转移能力的NL9980、H661、95C减少(图 1A); 同时, 细胞内FABP4蛋白表达水平在高转移能力的NL9980、H661、95C中较低转移能力的L9981、A549、PC13显著增加(图 1B、C)。
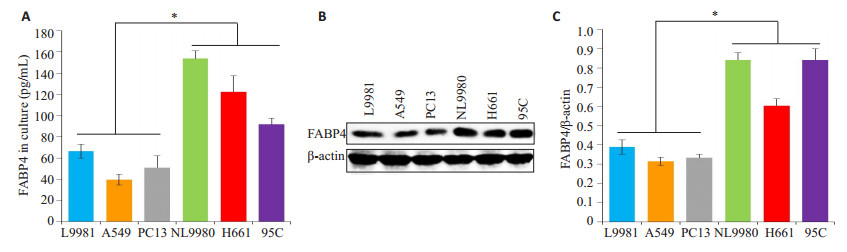
|
图 1 FABP4在肺癌细胞中的表达水平 Figure 1 Expression of FABP4 in lung cancer cells.A: ELISAresults; B, C: Western blotting results and quantitative analysis. *P < 0.05. |
通过shRNA技术降低高转移能力并高表达FABP4的NL9980细胞的FABP4表达后(图 2A、B), 其转移能力被显著抑制(图 2C、D); 而低转移能力并低表达FABP4的A549细胞中, 过表达FABP4后(图 2E、F), 其转移能力显著提高(图 2G、H)。
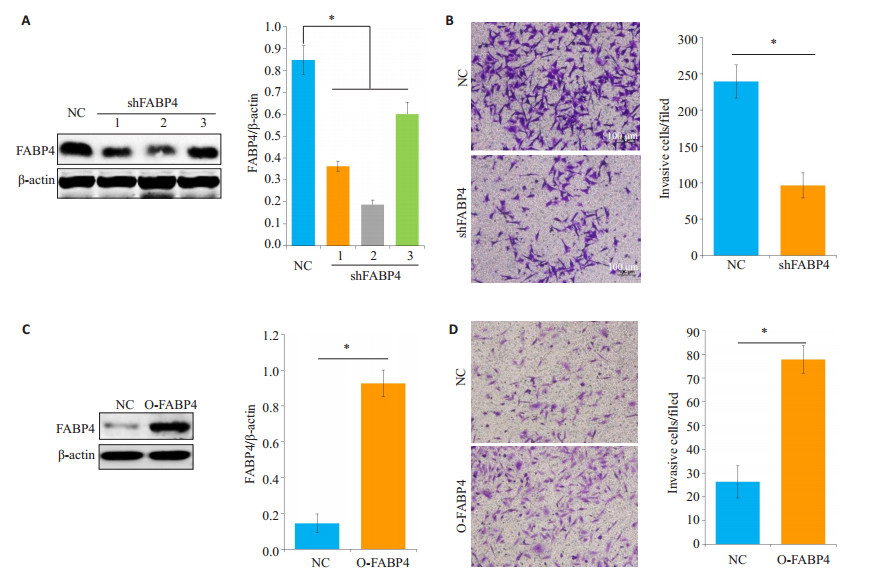
|
图 2 FABP4对肺癌细胞转移能力的影响 Figure 2 Effect of FABP4 on lung cancer cell metastasis. A: FABP4 expression detected by Western blotting in NL9980 cells transfected with shRNA; B: Transwell assay for assessing metastasis of NL9980 cells with FABP4 knockdown mediated by shFABP4- 2; C: Western blotting of FABP4 expression in A549 cells were transfected with FABP4-overexpressing lentivirus (O-FABP4); D: The metastasis of A549 cells with FABP4 overexpression was detected by Transwell assay. NC: Normal control. *P < 0.05. |
通过miRNA数据网站(http://www.microrna.org/)预测靶向FABP4的miRNA[14], 结果显示有miR-129、miR-155、miR-186、miR-203、miR-204、miR-205、miR-211、miR-361、miR-495和miR-539, 通过文献查询发[15]现miR-155 [15]、miR-205 [16]、miR-211 [17]和miR-495 [18]有促进肿瘤生长转移的作用, 而其他抑制肿瘤生长转移。结合FABP4促进肺癌转移, 而miRNA能靶向FABP4而抑制其表达的作用, 故选择检测抑制肿瘤生长转移的miR-129、miR-186、miR-203、miR-204、miR-361和miR-539。首先, Q-PCR检测发现, miR-203、miR-361和miR-539在高转移能力的肺癌细胞中表达较低转移的显著减少(图 3A~F)。用合成的miRNA模拟物转染NL9980细胞后, 发现miR-203、miR-204和miR-361能显著抑制FABP4的表达; 其中, miR-203的抑制效果最好(图 3G、H)。因此, 下一步实验用miR-203进行。通过miRNA数据网站(http://www.microrna.org/)预测miR-203靶向位点(图 3I), 构建miR-203靶向FABP4位点突变的带荧光素酶报告系统的质粒(MUT), 通过荧光素酶报告系统验证miR-203靶向FABP4的位点, 提示转染miR-203模拟物后, 靶向位点突变组荧光下降较对照组没有变化(图 3J)。
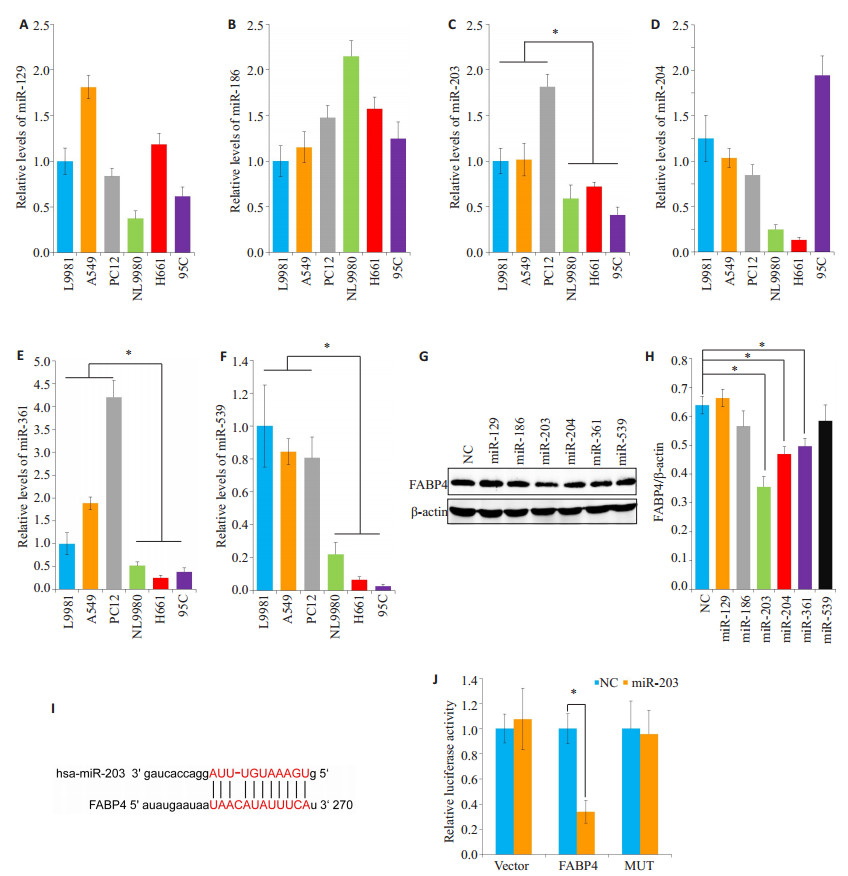
|
图 3 miR-203对肺癌细胞FABP4表达的影响 Figure 3 Effect of miR-203 on FABP4 expression in lung cancer cells. The expressions of miR-129 (A), miR-186 (B), miR-203 (C), miR-204 (D), miR-361 (E) and miR-539 (F) in lung cancer cells were tested by Q-PCR. G, H: FABP4 expression in NL9980 cells transfected with miRNA mimics; I: Prediction of miR-203 target sites via miRNA Data Website (http://www.microrna.org/); J: NL9980 cells transfected with the luciferase reporter system-targeting plasmid (MUT) for miR-203-mediated targeted FABP4 site mutation. *P < 0.05. |
NL9980细胞转染miR-203模拟物后, FABP4蛋白表达下降(图 4A), 其转移能力也显著下降(图 4B); 但转染靶位点突变的FABP4(MUT)后, NL9980细胞FABP4蛋白表达增加不能被miR-203抑制(图 4A), 同时miR-203过表达导致的转移能力下降也被抑制(图 4B)。而在低转移能力的A-549细胞转染miR-203抑制物后, FABP4蛋白表达增加(图 4C), 细胞转移能力显著增强(图 4D)。
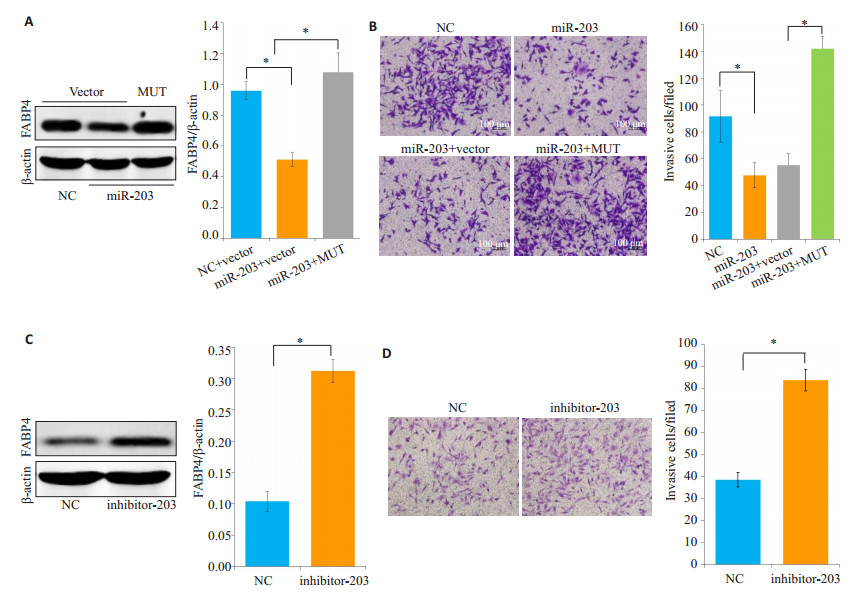
|
图 4 miR-203靶向FABP4抑制肺癌细胞的转移 Figure 4 miR-203 targets FABP4 to inhibit lung cancer cell metastasis. Mutant FABP4 (MUT) of the target site and miR-203 mimics were co-transfected into NL9980 cells, and the expression FABP4 (A and B) and metastasis (C and D) were detected by Western blotting and Transwell assay, respectively. After transfection of A549 cells with miR-203 inhibitor, Western blotting and Transwell assay were performed to detect the expression of FABP4 (E and F) and metastasis (G and H). NC: Normal control. Scale bar: 100 μm. *P < 0.05. |
FABP4是一类重要的脂类伴侣, 是脂质在细胞中代谢的重要蛋白[19]。研究发现, FABP4可提高乳腺癌细胞ROS水平促进其增殖[20], 也可诱导卵巢癌细胞EMT进而促进细胞的侵袭[6]; 大规模临床研究还发现FABP4与非小细胞肺癌[8]、胰腺导管癌[21]等的疾病进展有非常密切的关系。本研究通过检测肺癌细胞系FABP4表达水平, 发现低转移能力的L9981、A549、PC13培养基中FABP4的水平较高转移能力的NL9980、H661、95C低; 同时, 细胞内FABP4蛋白表达水平在高转移能力的NL9980、H661、95C中较低转移能力的L9981、A549、PC13显著增加。进一步在肺癌细胞系中降低或过表达FABP4, 都显著影响细胞的转移能力。由此说明, FABP4可调控肺癌细胞的转移。
miRNA是一类大小为20~25个核苷酸的非编码小RNA, 参与调节细胞的增殖、分化和凋亡等[22]。miRNA在肿瘤增殖、转移等中的作用已被大量证实[23-24]。既有研究发现miRNA在肺癌组织中表达增加, 可促进肺癌的增殖、侵袭转移[25]; 也有研究发现miRNA可抑制肿瘤发展重要蛋白的表达而抑制其增殖、侵袭转移, 但表达水平被显著抑制[26]。本研究发现可靶向FABP4的miR- 129、miR-186、miR-203、miR-204、miR-361和miR-539中miR-203、miR-361和miR-539的表达在高转移性的肺癌细胞中表达显著减少。过表达miR-203后可显著抑制NL9980的侵袭转移能力; 而抑制miR-203后, A549细胞的转移能力被显著增加, 这与他人在肺癌上的研究结果一致[27]。
进一步的结果还显示除了他人报道的miR-203除了靶向RGS17 [28]、LASP-1 [29]等来抑制肺癌的发生发展外, 本研究发现其还可以显著抑制FABP4的表达。肺癌细胞转染突变miR-203靶点的FABP4后, 其转移能力被miR-203抑制减少。由此提示, FABP4是miR-203抑制肺癌细胞转移的一个重点靶点。综上可以说明FABP4可以抑制肺癌细胞的转移, 而miR-203可以靶向FABP4抑制肺癌细胞的转移, 这也许能为肺癌转移的诊疗提供一个新的靶点。
| [1] |
陈万青, 郑荣寿, 张思维, 等. 2013年中国恶性肿瘤发病和死亡分析[J].
中国肿瘤, 2017, 26(1): 1-7.
|
| [2] |
Jin L, Chun J, Pan C, et al. The PLAG1-GDH1 axis promotes anoikis resistance and tumor metastasis through CamKK2-AMPK signaling in LKB1-Deficient lung cancer[J].
Mol Cell, 2018, 69(1): 87-99.
DOI: 10.1016/j.molcel.2017.11.025. |
| [3] |
毛楠, 何关生, 饶进军, 等. 沉默Bmi-1表达可逆转肺癌细胞对顺铂的耐药性[J].
南方医科大学学报, 2014(7): 1000-4.
|
| [4] |
Chang L, Zhang J, Liu L, et al. Fatty acid binding protein 4 is associated with stroke risk and severity in patients with acute ischemic stroke[J].
J Neuroimmunol, 2017, 311(7): 29-34.
|
| [5] |
Hertzel AV, Xu H, Downey M, et al. Fatty acid binding protein 4/aP2- dependent BLT1R expression and signaling[J].
J Lipid Res, 2017, 58(7): 1354-61.
DOI: 10.1194/jlr.M074542. |
| [6] |
Jin J, Zhang Z, Zhang S, et al. Fatty acid binding protein 4 promotes epithelial-mesenchymal transition in cervical squamous cell carcinoma through AKT/GSK3β/Snail signaling pathway[J].
Mol Cell Endocrinol, 2018, 461(4): 155-64.
|
| [7] |
Thompson KJ, Austin RG, Nazari SS, et al. Altered fatty acidbinding protein 4 (FABP4) expression and function in human and animal models of hepatocellular carcinoma[J].
Liver Int, 2017: 24.
DOI: 10.1111/liv.13639. |
| [8] |
Tang ZY, Shen Q, Xie H, et al. Elevated expression of FABP3 and FABP4 cooperatively correlates with poor prognosis in non-small cell lung cancer (NSCLC)[J].
Oncotarget, 2016, 7(29): 46253-62.
|
| [9] |
Castro D, Moreira M, Gouveia AM, et al. MicroRNAs in lung cancer[J].
Oncotarget, 2017, 8(46): 81679-85.
|
| [10] |
Zhou Q, Huang SX, Zhang F, et al. MicroRNAs: A novel potential biomarker for diagnosis and therapy in patients with non-small cell lung cancer[J].
Cell Prolif, 2017, 50(6): 35.
|
| [11] |
Wang Q, Shi G, Teng Y, et al. Successful reduction of inflammatory responses and arachidonic acid-cyclooxygenase 2 pathway in human pulmonary artery endothelial cells by silencing adipocyte fatty acid-binding protein[J].
J Inflamm (Lond), 2017, 14(8): 8.
|
| [12] |
Zhang Z, Liu X, Feng B, et al. STIM1, a direct target of microRNA- 185, promotes tumor metastasis and is associated with poor prognosis in colorectal cancer[J].
Oncogene, 2014, 34(37): 4808-20.
|
| [13] |
Hidaka H, Seki N, Yoshino H, et al. Tumor suppressive microRNA- 1285 regulates novel molecular targets: aberrant expression and functional significance in renal cell carcinoma[J].
Oncotarget, 2012, 3(1): 44-57.
|
| [14] |
Marchi S, Lupini L, Patergnani S, et al. Downregulation of the mitochondrial Calcium uniporter by cancer-related miR-25[J].
Curr Biol, 2013, 23(1): 58-63.
DOI: 10.1016/j.cub.2012.11.026. |
| [15] |
Gao Y, Liu Z, Ding Z, et al. MicroRNA-155 increases colon cancer chemoresistance to cisplatin by targeting forkhead box O3[J].
Oncol Lett, 2018, 15(4): 4781-8.
|
| [16] |
Tao Y, Song Y, Han T, et al. miR-205 regulation of ICT1 has an oncogenic potential via promoting the migration and invasion of gastric cancer cells[J].
Biomed Pharmacother, 2017, 96(8): 191-7.
|
| [17] |
Kang M, Shi J, Peng N, et al. MicroRNA-211 promotes non-smallcell lung Cancer proliferation and invasion by targeting MxA[J].
Onco Targets Ther, 2017, 10(7): 5667-75.
|
| [18] |
Wei T, Zhu W, Fang S, et al. miR-495 promotes the chemoresistance of SCLC through the epithelial-mesenchymal transition via Etk/ BMX[J].
Am J Cancer Res, 2017, 7(3): 628-46.
|
| [19] |
Xiong X, Zhang C, Zhang Y, et al. Fabp4-Cre-mediated Sirt6 deletion impairs adipose tissue function and metabolic homeostasis in mice[J].
J Endocrinol, 2017, 233(3): 307-14.
DOI: 10.1530/JOE-17-0033. |
| [20] |
Guaita-Esteruelas S, Bosquet A, Saavedra P, et al. Exogenous FABP4 increases breast cancer cell proliferation and activates the expression of fatty acid transport proteins[J].
Mol Carcinog, 2017, 56(1): 208-17.
DOI: 10.1002/mc.v56.1. |
| [21] |
Luo Y, Yang Z, Li D, et al. LDHB and FABP4 are Associated with progression and poor prognosis of pancreatic ductal adenocarcinomas[J].
Appl Immunohistochem Mol Morphol, 2017, 25(5): 351-7.
DOI: 10.1097/PAI.0000000000000306. |
| [22] |
Schueller F, Roy S, Vucur M, et al. The role of miRNAs in the pathophysiology of liver diseases and toxicity[J].
Int J Mol Sci, 2018, 19(1): 78.
|
| [23] |
Shenouda SK, Alahari SK. MicroRNA function in cancer: oncogene or a tumor suppressor[J].
Cancer Metastasis Rev, 2009, 28(3/4): 369-78.
|
| [24] |
Trang P, Weidhaas JB, Slack FJ. MicroRNAs as potential cancer therapeutics[J].
Oncogene, 2008, 27(Suppl 2): S52-7.
|
| [25] |
Tang YT, Cui YY, Li ZP, et al. Radiation-induced miR-208a increases the proliferation and radioresistance by targeting p21 in human lung cancer cells[J].
J Exp Clin Cancer Res, 2016, 35(11): 7.
|
| [26] |
Xia M, Duan ML, Tong JH, et al. MiR- 26b suppresses tumor cell proliferation, migration and invasion by directly targeting COX-2 in lung cancer[J].
Eur Rev Med Pharmacol Sci, 2015, 19(24): 4728-37.
|
| [27] |
Zheng XB, Chen XB, Xu LL, et al. miR-203 inhibits augmented proliferation and metastasis of hepatocellular carcinoma residual in the promoted regenerating liver[J].
Cancer Sci, 2017, 108(3): 338-46.
DOI: 10.1111/cas.13167. |
| [28] |
Chi Y, Jin Q, Liu X, et al. miR-203 inhibits cell proliferation, invasion, and migration of non-small-cell lung cancer by downregulating RGS17[J].
Cancer Sci, 2017, 108(12): 2366-72.
DOI: 10.1111/cas.2017.108.issue-12. |
| [29] |
Zheng J, Wang F, Lu S, et al. LASP-1, regulated by miR-203, promotes tumor proliferation and aggressiveness in human nonsmall cell lung cancer[J].
Exp Mol Pathol, 2016, 100(1): 116-24.
DOI: 10.1016/j.yexmp.2015.11.031. |
 2018, Vol. 38
2018, Vol. 38

