2. 国立癌症研究所肿瘤生物与遗传实验室, 国立卫生研究院, 马里兰州贝塞斯达市, 美国
2. Laboratory of Cancer Biology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA
PD-L1 (B7-H1, CD274)是PD-1的配体, 在多种细胞中均有表达, 包括肿瘤细胞、单核细胞、巨噬细胞、T细胞和树突状细胞。PD-L1与PD-1结合并激活PD-L1/ PD-1信号通路, 在不同的细胞中导致不同的结果。激活T细胞的PD-L1/PD-1通路可抑制T细胞增殖、减少T细胞INF-γ和IL-12的分泌、导致T细胞衰竭和凋亡, 从而导致T细胞的免疫抑制。癌细胞表达PD-L1抑制T细胞功能是癌症患者肿瘤细胞逃避免疫监视的主要机制, 因此, 用肿瘤细胞表面表达PD-L1作为选择接受PD-1或PD-L1抗体治疗的癌症患者的标志[1-6]。虽然有研究表明其他免疫细胞, 如树突状细胞[7-10]表面也表达PD-1, 但和T细胞相比, PD-L1/PD-1通路激活对其它免疫细胞影响的研究却很少。
树突状细胞是最强的抗原提呈细胞, 它由骨髓祖细胞或单核细胞[11]分化而来。在肿瘤微环境中, 树突状细胞的成熟是受到抑制的[12]。肿瘤组织中未成熟树突状细胞数量多提示预后较差。有研究认为树突状细胞的成熟抑制是由肿瘤细胞分泌的细胞因子所引起的[11-17]。未成熟树突状细胞通过多种机制引起肿瘤微环境中T细胞耐受[11]。除了对T细胞的影响, 有研究认为, 肿瘤相关树突状细胞可以通过分泌HB-EGF、CXCL5或双调蛋白促进肿瘤细胞的侵袭[18-20]。免疫组化结果显示, 在肺癌组织中树突状细胞的不成熟与癌细胞PD-L1的表达相关[21]。Lim等[22]研究结果表明树突状细胞表面表达的PD-1可以抑制CD8+T细胞的功能和其抗肿瘤免疫的作用。然而, PD-L1/PD-1信号通路在树突状细胞分化成熟中起何作用未见报道。
基于以上研究背景, 本研究拟探讨表达在乳腺癌细胞细胞膜的PD-L1是否通过激活树突状细胞PD-L1/ PD-1信号通路抑制树突状细胞成熟。
1 材料和方法 1.1 材料 1.1.1 细胞及其培养相关试剂MDA-MB-231、MCF-7和T47-D细胞系(ATCC); DMEM、RPMI 1640、L-15、胎牛血清、青霉素、链霉素和L-谷氨酰胺(Gibco); 人单个核细胞, 从健康志愿者新鲜血液分离; 细胞示踪剂CMFDA (Abcam)。
1.1.2 人单核细胞分离纯化与树状突细胞诱导分化有关试剂淋巴细胞分离液(灏洋华科); 抗CD14+的磁珠(美天旎); 细胞因子IL-4、GM-CSF和TNF-α (R & D)。
1.1.3 抗体与重组人PD-L1蛋白PD-L1抗体及同型对照抗体(Biolegend); PD-L1-APC抗体及同型对照抗体(e-Bioscience); CD83-APC、HLA-DR-PE以及同型对照抗体(BD); 人重组PD-L1蛋白(Sino Biological)。
1.2 方法 1.2.1 乳腺癌细胞系与细胞培养MCF-7和T47-D细胞培养于DMEM, MDA-MB-231细胞培养于L-15, 含10%胎牛血清、100 U/mL青霉素、100 μg/mL链霉素以及2 mmol/L谷氨酰胺, 细胞培养于37 ℃含5% CO2的培养箱中。
1.2.2 分离人外周血单核细胞及体外诱导树突状细胞健康志愿者新鲜血液分离, 用肝素抗凝, 用淋巴细胞分离液通过密度梯度离心法分离人单个核细胞(PBMC), PBMC再用抗CD14+的磁珠分选出单核细胞, 单核细胞培养于RPMI 1640中, 含10%胎牛血清及2 mmol/L谷氨酰胺, 在37 ℃含5% CO2的培养箱中。单核细胞用IL-4与GM-CSF诱导为未成熟树突状细胞, 再加入TNF-α诱导为成熟的树突状细胞[23]。单核细胞以1×105/孔的密度种于12孔板, 于第1天加入细胞因子IL-4 (40 ng/mL)与GM-CSF (40 ng/mL); 于第3天更换新鲜培养基以及细胞因子; 在第4天得到未成熟树突状细胞, 再加入TNF-α (40 ng/mL)继续培养2 d, 于第6天得到成熟的树突状细胞。
1.2.3 细胞示踪剂CMFDA预染MDA-MB-231细胞并与未成熟树突状细胞共培养当MDA-MB-231细胞生长密度达到80%后, 吸出培养基, 加入含0.5µmol细胞示踪剂CMFDA[24-25]的预热培养基, 预染乳腺癌细胞45 min, 而后弃去染液, 更换为新鲜培养基, MDA-MB- 231细胞继续培养30 min。预染后的MDA-MB-231细胞用胰酶消化为单个细胞, 按1: 1的比例和未成熟树突状细胞共培养48 h, 培养基中含有IL-4、GM-CSF和TNF-α, 用胰酶消化共培养的细胞, 并用流式细胞术检测树突状细胞群表面标记。
1.2.4 PD-L1抗体与共培养MDA-MB-231和未成熟树突状细胞的孵育以及人重组PD-L1蛋白处理单独培养的树突状细胞在共培养的预染MDA-MB-231细胞与未成熟树突状细胞中加入PD-L1抗体(10 µg/mL)、同型对照抗体(10 µg/mL)和TNF-α, 继续培养48 h。单独培养的单核细胞由IL-4及GM-CSF诱导4 d成为未成熟树突状细胞, 再加入TNF-α和人重组PD-L1蛋白(终浓度在2 µg/mL) [26], 继续培养48 h。
1.2.5 流式细胞术检测细胞膜表面蛋白用流式细胞术检测肿瘤细胞膜表面表达的PD-L1蛋白以及树突状细胞表面的CD83和HLA-DR。流式细胞仪检测的细胞用流式细胞仪缓冲液洗2遍, 随后在4 ℃与荧光抗体(PD-L1-APC、CD83-APC或HLA-DR-PE抗体)或同型对照抗体孵育30 min; 1000 r/min 4 ℃离心细胞5 min弃去多余抗体并加入200 µL流式细胞仪缓冲液, 用BD CytoFLEX流式细胞仪进行检测, 检测结果用FLowJo进行分析。
1.2.6 统计分析所有实验均重复3次, 数据表达为均数±标准差。应用SPSS21.0软件进行统计分析, 两组数据之间的差异比较用独立样本t检验, 多组数据之间的差异比较应用ANOVA统计方法。P < 0.05视为差异有统计学意义。
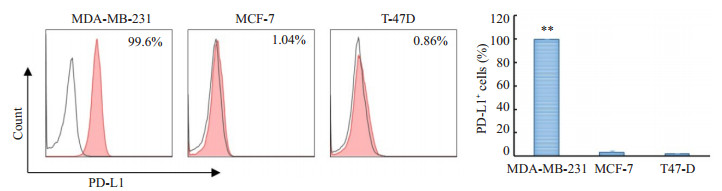
2 结果 2.1 3个乳腺癌细胞系细胞表面PD-L1蛋白表达的百分率在3种细胞系中, MDA-MB-231细胞表达PD-L1的阳性细胞高达99.7±0.15%, 相比之下MCF-7与T47D分别为(2.85±1.77) %和(1.44±0.51) % (图 1)。
|
图 1 3个乳腺癌细胞系细胞膜表面PD-L1蛋白表达的百分率 Figure 1 Percentages of PD-L1 expression on cell membrane of the 3 breast cancer cell lines (**P < 0.01 vs MCF-7, T47-D). |
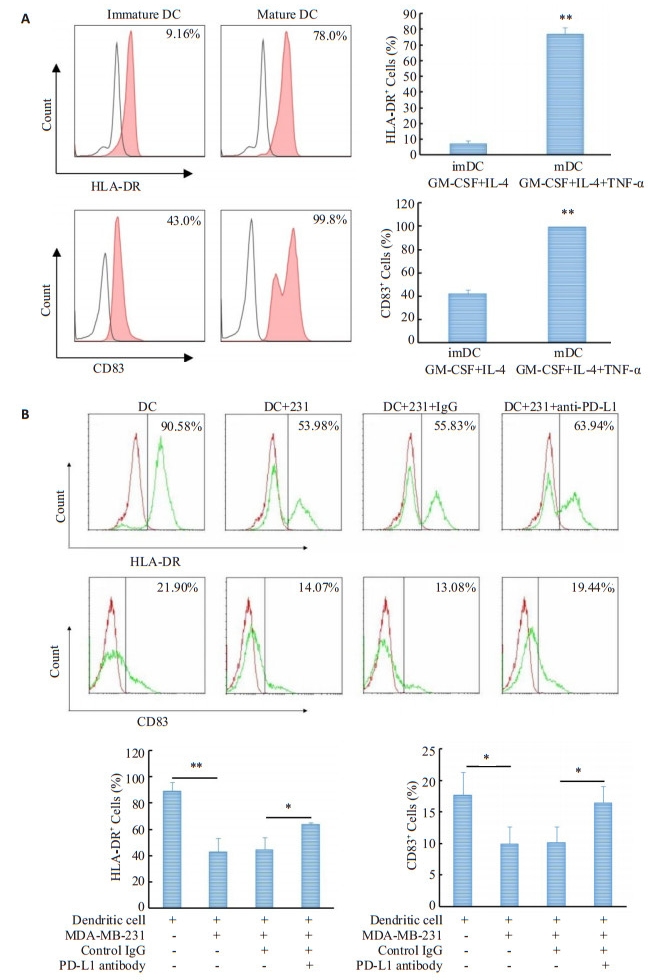
人单核细胞在IL-4(40 ng/mL)和GM-CSF(40 ng/mL)诱导4d后可分化为不成熟的树突状细胞, 其特征为低表达CD83与HLA-DR; 加入TNF-α (40 ng/mL)继续诱导2d, 树突状细胞分化成熟, 其特征为两种表面标记的阳性细胞百分比均有上升, HLA-DR由(7.18± 1.73) %上升至(76.8±4.13) %, CD83由(41.77±3.61) %上升至(99.37±0.40) % (图 2A)。
|
图 2 MDA-MB-231细胞对共培养树突状细胞成熟分化的影响及PD-L1抗体对这一影响的作用 Figure 2 Effect of MDA-MB-231 cells on maturation of co-cultured dendritic cells and the impact of PD-L1 antibody on this effect. A: Percentages of HLA-DR- and CD83-positive cells in immature and mature dendritic cells (**P < 0.01 vs imDC); B: Effects of MDA-MB-231 cells on percentages of HLADR- and CD83-positive cells in co-cultured dendritic sub-population cells and the impact of PD-L1 antibody on these effects (**P < 0.01, *P < 0.05). |
流式细胞仪分析表明, 共培养的两种细胞可被区分为两个细胞群(数据未展示), 一群为预染CMFDA的MDA-MB-231细胞, 另一群为单核细胞诱导而来的树突状细胞。CD83和HLA-DR的阳性细胞百分率只在树突状细胞群中进行分析。和成熟树状突细胞对照组相比, 共培养细胞中树状突细胞群HLA-DR和CD83阳性细胞的百分率下降, HLA-DR由(88.8±6.96) %降至(42.76±10.52) %, CD83由(18.36±3.07) %降至(9.93± 2.74) % (图 2B)。
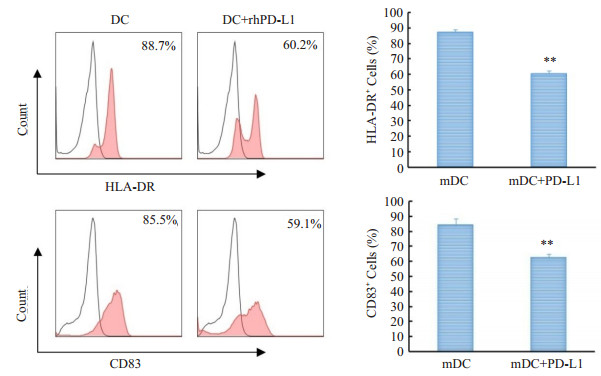
和同型对照组相比, PD-L1抗体处理组树状突细胞亚群HLA-DR和CD83阳性细胞的百分比下降, HLADR阳性细胞百分比由(45.17±10.19) %升至(63.46± 1.72)%, CD83的阳性细胞百分比由(10.15±2.54) %升至(16.46±2.58) % (图 2B), 统计结果显示PD-L1抗体组与同型对照组相比HLA-DR和CD83的阳性细胞百分比均有统计学意义(P < 0.05)。2.3人重组PD-L1蛋白对树突状细胞成熟分化的影响流式细胞术检测结果(图 3)显示, 和成熟树状突细胞对照组比, 人重组PD-L1蛋白处理可以降低树状突细胞HLA-DR和CD83阳性细胞的百分率, HLA-DR由(84.23±4.18) %降至(62.56±2.39) %, CD83由(87.26± 1.54) %降至(60.67±1.63)% (P < 0.01)。
|
图 3 人重组PD-L1蛋白对熟树突状细胞成熟分化的影响 Figure 3 Effect of recombinant human PD-L1 protein on maturation differentiation of dendritic cells (**P < 0.01 vs mDC). |
虽然肿瘤微环境中树突状细胞成熟受到抑制的机制已有报道, 但对这一机制的认识并无定论。本研究表明, PD-L1抗体可以减弱表达PD-L1的乳腺癌细胞MDA-MB-231对共培养树突状细胞成熟分化的抑制作用, 而人重组PD-L1蛋白可抑制单核细胞来源的未成熟树突状细胞向成熟分化, 这些结果提示, PD-1信号通路的激活可抑制TNF-α诱导未成熟树突状细胞向成熟方向分化的过程。
PD-L1在多种肿瘤细胞表面均有表达, 其中包括乳腺癌细胞[2, 27-28], PD-L1抗体也对多种实体瘤的治疗有效果[3, 29]。临床实验结果表明表达PD-L1的三阴性乳腺癌对PD-L1抗体的治疗有效应[2, 30-31], 疾病控制率达到25%。有研究报道乳腺癌细胞系MDA-MB-231细胞高表达PD-L1蛋白[32-33], 本实验的数据与之前的报道一致, 即MDA-MB-231细胞膜表面高表达PD-L1蛋白, 而乳腺癌细胞系MCF-7与T47D基本不表达。有报道指出, 肿瘤微环境中T细胞分泌的INF-γ上调乳腺癌细胞表达的PD-L1 [34-35]。单独培养的MDA-MB-231细胞表面表达PD-L1, 说明肿瘤细胞膜表面组成性表达PD-L1可不依赖与肿瘤微环境中T细胞分泌的INF-γ, 反而, 肿瘤细胞通过组成性表达的PD-L1抑制T细胞、巨噬细胞和树突状细胞。
在本实验模型中, CD14+人单核细胞可以被IL-4和GM-CSF诱导为不成熟的树突状细胞, 再加入TNF-α则可诱导分化为成熟的树突状细胞; 成熟的树突状细胞HLA-DR和CD83的阳性细胞比例较高。在MDAMB-231细胞与未成熟树突状细胞的接触共培养实验中, 发现即使加入了TNF-α, MDA-MB-231细胞仍可以抑制树突状细胞的成熟, 而加入PD-L1抗体后可以减弱这一抑制作用, 部分恢复树突状细胞向成熟分化, 可能的机制是, PD-L1抗体改变了树突状细胞和/或肿瘤细胞分泌的细胞因子, 但另一种可能性是PD-L1抗体阻断了MDA-MB-231细胞的PD-L1, 使其不能通过PD-1信号通路影响与其共培养的未成熟树突状细胞, 从而减弱了MDA-MB-231细胞对树突状细胞分化成熟的抑制作用。事实上, Mu等[21]的研究表明, 肺癌组织切片中, 未成熟树突状细胞与PD-L1的表达相关, 虽然他们没有提供更多的数据证明PD-L1可以向树突状细胞传递信号从而抑制树突状细胞的成熟分化。因有多篇论文报道树突状细胞膜表面表达PD-1[7-9, 36], 所以有理由认为, PD-1信号途径的激活抑制TNF-α诱导的未成熟的树突状细胞向成熟分化。
为了更进一步证明本研究的假设, 即PD-L1激活树突状细胞的PD-1信号通路从而抑制树突状细胞成熟, 我们将未成熟树突状细胞与人重组PD-L1共孵育, 观察人重组PD-L1对未成熟树突状细胞的影响。我们的数据支持本研究的假设, 即人重组PD-L1可以抑制TNF-α诱导单核细胞来源的未成熟树突状细胞向成熟分化的过程。
基于本研究的数据, 提出乳腺癌细胞表面表达的PD-L1可通过D-L1/PD-1信号通路抑制肿瘤微环境中的树突状细胞成熟, 因此导致肿瘤微环境中的树突状细胞不能提呈抗原或使不成熟树状突细胞行使其他功能。PD-L1抗体对三阴性乳腺癌患者疗效的部分原因是抗体可以阻断乳腺癌细胞表达的PD-L1, 从而削弱其对肿瘤微环境中树突状细胞成熟的抑制作用。
| [1] |
Tomioka N, Azuma M, Ikarashi M, et al. The therapeutic candidate for immune checkpoint inhibitors elucidated by the status of tumorinfiltrating lymphocytes (TILs) and programmed death ligand 1 (PDL1) expression in triple negative breast cancer (TNBC)[J].
Breast Cancer, 2018, 25(1): 34-42.
DOI: 10.1007/s12282-017-0781-0. |
| [2] |
Bertucci F, Gonçalves A. Immunotherapy in breast cancer: the emerging role of PD-1 and PD-L1[J].
Curr Oncol Rep, 2017, 19(10): 64.
DOI: 10.1007/s11912-017-0627-0. |
| [3] |
Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients[J].
Nature, 2014, 515(7528): 563-7.
DOI: 10.1038/nature14011. |
| [4] |
Sacher AG, Gandhi L. Biomarkers for the clinical use of PD-1/PDL1 inhibitors in Non- Small- Cell lung cancer: a review[J].
JAMA oncol, 2016, 2(9): 1217-22.
DOI: 10.1001/jamaoncol.2016.0639. |
| [5] |
Smit EF, Van Den Heuvel MM. PD-L1 in non-small-cell lung cancer: the third target for immunotherapy[J].
Lancet, 2016, 387(10030): 1795-6.
DOI: 10.1016/S0140-6736(16)00700-5. |
| [6] |
Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti- PD-1 therapy[J].
Clin Cancer Res, 2014, 20(19): 5064-74.
DOI: 10.1158/1078-0432.CCR-13-3271. |
| [7] |
Yao S, Wang S, Zhu Y, et al. PD-1 on dendritic cells impedes innate immunityagainstbacterialinfection[J].
Blood, 2009, 113(23): 5811-8.
DOI: 10.1182/blood-2009-02-203141. |
| [8] |
Probst HC, Mccoy K, Okazaki T, et al. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4[J].
Nat Immunol, 2005, 6(3): 280-6.
DOI: 10.1038/ni1165. |
| [9] |
Van Der Waart AB, Fredrix H, Van Der Voort R, et al. siRNA silencing of PD-1 ligands on dendritic cell vaccines boosts the expansion of minor histocompatibility antigen- specific CD8(+) T cells in NOD/SCID/IL2Rg(null) mice[J].
Cancer Immunol Immunother, 2015, 64(5): 645-54.
DOI: 10.1007/s00262-015-1668-6. |
| [10] |
Karyampudi L, Lamichhane P, Krempski J, et al. PD- 1 blunts the function of ovarian Tumor-Infiltrating dendritic cells by inactivating NF-κB[J].
Cancer Res, 2016, 76(2): 239-50.
DOI: 10.1158/0008-5472.CAN-15-0748. |
| [11] |
Tang M, Diao J, Cattral MS. Molecular mechanisms involved in dendritic cell dysfunction in cancer[J].
Cell Mol Life Sci, 2017, 74(5): 761-76.
DOI: 10.1007/s00018-016-2317-8. |
| [12] |
Tran Janco JM, Lamichhane P, Karyampudi L, et al. Tumorinfiltrating dendritic cells in cancer pathogenesis[J].
J Immunol, 2015, 194(7): 2985-91.
DOI: 10.4049/jimmunol.1403134. |
| [13] |
Michielsen AJ, O'sullivan JN, Ryan EJ. Tumor conditioned media from colorectal cancer patients inhibits dendritic cell maturation[J].
Oncoimmunology, 2012, 1(5): 751-3.
DOI: 10.4161/onci.19570. |
| [14] |
Veglia F, Gabrilovich DI. Dendritic cells in cancer: the role revisited[J].
Curr Opin Immunol, 2017, 45: 43-51.
DOI: 10.1016/j.coi.2017.01.002. |
| [15] |
Ma Y, Shurin GV, Zhu PY, et al. Dendritic cells in the cancer microenvironment[J].
J Cancer, 2013, 4(1): 36-44.
DOI: 10.7150/jca.5046. |
| [16] |
Michielsen AJ, Hogan AE, Marry J, et al. Tumour tissue microenvironment can inhibit dendritic cell maturation in colorectal cancer[J].
PLoS One, 2011, 6(11): e27944.
DOI: 10.1371/journal.pone.0027944. |
| [17] |
Dudek AM, Martin S, Garg AD, et al. Immature, Semi-Mature, and fully mature dendritic cells: toward a DC-Cancer cells interface that augments anticancer immunity[J].
Front Immunol, 2013, 4: 438.
|
| [18] |
Kuo PL, Huang MS, Hung JY, et al. Synergistic effect of lung tumorassociated dendritic cell- derived HB-EGF and CXCL5 on cancer progression[J].
Int J Cancer, 2014, 135(1): 96-108.
DOI: 10.1002/ijc.v135.1. |
| [19] |
Hsu YL, Huang MS, Cheng DE, et al. Lung tumor- associated dendritic cell-derived amphiregulin increased cancer progression[J].
J Immunol, 2011, 187(4): 1733-44.
DOI: 10.4049/jimmunol.1100996. |
| [20] |
Kuo CH, Chen KF, Chou SH, et al. Lung tumor-associated dendritic cell-derived resistin promoted cancer progression by increasing Wolf-Hirschhorn syndrome candidate 1/Twist pathway[J].
Carcinogenesis, 2013, 34(11): 2600-9.
DOI: 10.1093/carcin/bgt281. |
| [21] |
Mu CY, Huang JA, Chen Y, et al. High expression of PD-L1 in lung cancer May contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation[J].
Med Oncol, 2011, 28(3): 682-8.
DOI: 10.1007/s12032-010-9515-2. |
| [22] |
Lim TS, Chew V, Sieow JL, et al. PD-1 expression on dendritic cells suppresses CD8 + T cell function and antitumor immunity[J].
Oncoimmunology, 2016, 5(3): e1085146.
DOI: 10.1080/2162402X.2015.1085146. |
| [23] |
LopesAMM, Michelin MA, Murta EFC. Monocyte-derived dendritic cells from patients with cervical intraepithelial lesions[J].
Oncol Lett, 2017, 13(3): 1456-62.
DOI: 10.3892/ol.2017.5595. |
| [24] |
Król M, Pawłowski KM, Majchrzak K, et al. Global gene expression profiles of canine macrophages and canine mammary cancer cells grown as a co-culture in vitro[J].
BMC Vet Res, 2012, 8: 16.
DOI: 10.1186/1746-6148-8-16. |
| [25] |
Hollmén M, Roudnicky F, Karaman S, et al. Characterization of macrophage--cancer cell crosstalk in estrogen receptor positive and triple-negative breast cancer[J].
Sci Rep, 2015, 5: 9188.
DOI: 10.1038/srep09188. |
| [26] |
Zhou ZQ, Tong DN, Guan J, et al. Follicular helper T cell exhaustion induced by PD-L1 expression in hepatocellular carcinoma results in impaired cytokine expression and B cell help, and is associated with advanced tumor stages[J].
Am J Transl Res, 2016, 8(7): 2926-36.
|
| [27] |
Mittendorf EA, PhilipsAV, Meric-Bernstam F, et al. Pd-l1 expression in triple-negative breast cancer[J].
Cancer Immunol Res, 2014, 2(4): 361-70.
DOI: 10.1158/2326-6066.CIR-13-0127. |
| [28] |
Baptista MZ, Sarian LO, Derchain SF, et al. Prognostic significance of PD-L1 and PD-L2 in breast cancer[J].
Hum Pathol, 2016, 47(1): 78-84.
DOI: 10.1016/j.humpath.2015.09.006. |
| [29] |
Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of antiPD-L1 antibody in patients with advanced cancer[J].
N Engl J Med, 2012, 366(26): 2455-65.
DOI: 10.1056/NEJMoa1200694. |
| [30] |
Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in patients with advanced Triple-Negative breast cancer: phase Ib KEYNOTE-012 study[J].
J Clin Oncol, 2016, 34(21): 2460-7.
DOI: 10.1200/JCO.2015.64.8931. |
| [31] |
Hartkopf AD, Taran FA, Wallwiener M, et al. PD-1 and PD- L1 immune checkpoint blockade to treat breast cancer[J].
Breast Care (Basel), 2016, 11(6): 385-90.
DOI: 10.1159/000453569. |
| [32] |
Soliman H, Khalil F, Antonia S. PD-L1 expression is increased in a subset of basal type breast cancer cells[J].
PLoS One, 2014, 9(2): e88557.
DOI: 10.1371/journal.pone.0088557. |
| [33] |
Coombs MR, Harrison ME, Hoskin DW. Apigenin inhibits the inducible expression of programmed death ligand 1 by human and mouse mammary carcinoma cells[J].
Cancer Lett, 2016, 380(2): 424-33.
DOI: 10.1016/j.canlet.2016.06.023. |
| [34] |
Mimura K, Teh JL, Okayama H, et al. PD-L1 expression is mainly regulated by interferon gamma associated with JAK-STAT pathway in gastric cancer[J].
Cancer Sci, 2018, 109(1): 43-53.
DOI: 10.1111/cas.2018.109.issue-1. |
| [35] |
Abiko K, Matsumura N, Hamanishi J, et al. IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer[J].
Br J Cancer, 2015, 112(9): 1501-9.
DOI: 10.1038/bjc.2015.101. |
| [36] |
Park SJ, Namkoong H, Doh J, et al. Negative role of inducible PD-1 on survival of activated dendritic cells[J].
J Leukoc Biol, 2014, 95(4): 621-9.
DOI: 10.1189/jlb.0813443. |
 2018, Vol. 38
2018, Vol. 38

