2. 海南省人民医院内分泌代谢科,海南 海口 570100
2. Department of Endocrinology and Metabolism, Hainan General Hospital, Haikou 570100, China
近30年来,全球糖尿病发病率显著增加。心血管并发症是糖尿病患者死亡的主要原因,其中,糖尿病心肌病(DCM)是一种独立于高血压、冠心病或其他心血管疾病的特异性心肌病,病理表现为心肌肥厚和心肌纤维化[1]。DCM的发病机制复杂,目前认为心肌线粒体功能障碍以及氧化应激在糖尿病心肌损伤中起关键作用[2]。
胰高血糖素样肽-1(GLP-1)是肠道L细胞分泌的一种多功能激素,除了控制血糖[3]、抑制胃排空[4]、降低食欲[5],还表现出对心血管疾病的保护作用[6]。动物研究发现GLP-1能够改善心肌梗死后左心室功能,预防动脉粥样硬化进展[7-9]。GLP-1受体在胰腺及胰腺外组织包括心血管系统中广泛表达[10-12]。Exendin-4是GLP-1受体激动剂,与GLP-1受体结合后产生与GLP-1相似的生理效应。国外有研究报道GLP-1能够改善糖尿病动物心肌的氧化应激、脂质代谢,逆转心脏重塑[13],但具体机制仍未完全阐明。因此,本研究利用高脂饮食联合链脲佐菌素(STZ)诱导建立2型糖尿病小鼠模型,通过观察exendin-4干预后小鼠心肌组织分子代谢及形态学变化,探讨可能的机制。
1 材料和方法 1.1 实验动物无特定病原体(SPF)级健康雄性C57BL/6J小鼠26只,5~6周龄,体质量18~25 g,由广东省实验动物中心提供。分笼饲养于南方医科大学SPF级动物房。室温保持在25±2 ℃,湿度保持在(55±5)%。动物自由进食,自由饮水,每天保持12 h的昼夜循环。所有实验操作符合科技部与南方医科大学动物实验伦理委员会的要求。
1.2 主要试剂链脲佐菌素(S0130, Sigma);高脂饲料(D12492,广东省实验动物中心);Exendin-4(E7144, sigma);兔源一抗PGC1α(Novus Biologicals, USA),兔源一抗Sirt1 (Abcam, USA),兔源一抗NOX1(GeneTex, USA),鼠源一抗SOD1(Santa Cruz Biotechnology);鼠源一抗β- actin(中杉金桥);羊抗兔IgG抗体、羊抗小鼠IgG抗体(proteintech);甘油三酯及胆固醇试剂盒;细胞裂解液、BCA蛋白定量试剂盒(凯基生物)
1.3 糖尿病小鼠模型的制备健康雄性C57BL/6J小鼠26只随机分为正常组(Con,6只)和糖尿病组(DM,20只),Con组小鼠正常饮食,DM组小鼠进行造模。DM组小鼠进行高脂饲料喂养4周,然后按照120 mg/kg体质量腹腔注射STZ 1次(12 mg/mL STZ溶于柠檬酸缓冲液,pH=4.2~4.5),继续饲喂高脂饲料4周后随机血糖值≥13.9 mmol/L (250 mg/dl)判定为2型糖尿病小鼠[14]。
1.4 动物分组,给药及标本采集将造模成功的16只小鼠再随机分为2组,每组8只,分别是糖尿病对照组(DM-Con),exendin-4干预组(DM+Ex-4)。DM+Ex-4组小鼠给予1 nmol/kg体质量的exendin-4腹腔注射,每天注射1次,重复注射8周,DM-Con组给予等剂量的生理盐水腹腔注射[15-16]。同时两组小鼠继续喂养高脂饲料。实验期间观察小鼠精神状态和活动情况,每周测量体质量、进食量、随机血糖。实验期间糖尿病小鼠均未予胰岛素治疗。给药8周后,戊巴比妥钠麻醉后处死小鼠,收集小鼠血液、心脏组织标本。
1.5 血清血脂检测将收集的小鼠血液3000 r/min低温离心10 min,取血清保存于负80 ℃冰箱,按照试剂盒说明书使用化学发光法检测血清甘油三酯(TG)、低密度脂蛋白胆固醇(LDL-c)。
1.6 实时荧光定量PCR(RT-PCR)Trizol法提取小鼠心脏组织总RNA,应用ND- 1000分光光度计(NanDrop,USA)测定RNA浓度和纯度。取等量(500 ng)RNA逆转录成cDNA。RT-qPCR扩增体系:SYBR Premix Ex TaqTM酶5 μL,上下游引物各0.2 μL,cDNA 1 μL,去离子水3.6 μL。在LC480实时荧光定量PCR仪中,按以下条件进行PCR反应:预变性:95 ℃ 30 s;PCR反应:95 ℃ 5 s,60 ℃ 34 s,进行40个循环。反应结束后常规进行溶解曲线分析,确定PCR扩增特异性,得出Ct后使用2-ΔΔCt法进行相对定量。以18 s为内参,计算目的基因的mRNA的相对表达量,引物序列见表 1。
| 表 1 引物序列 Table 1 Primer sequence |
将少量心脏组织加入500 μL的RIPA裂解液,匀浆机匀浆,离心后提取蛋白。BCA法测定蛋白浓度,取30 μg蛋白样品上样,进行SDS-PAGE电泳,转膜,5%脱脂奶粉室温封闭,一抗(稀释浓度分别是β-actin 1:200,Sirt1 1:1000,PGC1α 1:1000,NOX1 1:500,SOD1 1:200) 4 ℃孵育过夜,洗膜,分别加入1:10 000稀释的羊抗兔二抗、羊抗小鼠二抗,室温孵育1 h,再次洗膜后,浸入曝光液并吹打均匀上机曝光,并对曝光结果进行灰度值统计分析。
1.8 病理学检查心脏组织用4%中多聚甲醛溶液固定后,常规脱水、透明、浸蜡、包埋、石蜡切片,进行HE染色后光镜下观察心脏组织形态学变化。
1.9 统计学方法数据均以均数±标准差表示,采用SPSS 20.0统计软件进行处理。两组间比较采用t检验。多组均数间比较采用单因素方差分析,组间多重比较采用LSD-t检验。以P < 0.05为差异有统计学意义。
2 结果 2.1 2型糖尿病小鼠模型的建立饲养期间,Con组小鼠饮食正常,精神状态良好,毛发乌黑有光泽,反应灵敏。DM组小鼠经过高脂饲养2周后,体质量比Con组显著增高(P < 0.01)。STZ注射后,小鼠逐渐出现多饮、多食、多尿等糖尿病症状,小鼠精神萎靡,毛发蓬松,腥臊酮臭味加重,活动度下降,体质量增加不明显,于第7周始,体质量较Con组无显著性差异(P>0.05)。DM组小鼠于第6周始随机血糖明显高于Con组(P < 0.001),2型糖尿病小鼠造模成功(图 1)。
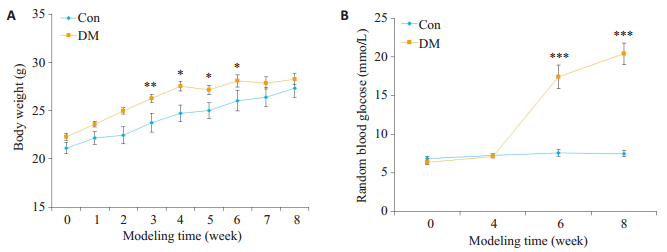
|
图 1 小鼠造模期间体质量和血糖的变化 Figure 1 Changes of body weight (A) and blood glucose (B) during the generation of type 2 diabetic mouse model (Mean±SE, n=6-8).*P < 0.05, **P < 0.01, ***P < 0.001 vs Con. |
造模成功后,DM+Ex-4组小鼠每日给予1 nmol/kg体质量的exendin-4腹腔注射,实验观察到DM+Ex-4组小鼠多饮多尿症状较DM-Con组有所改善,精神状态好转,毛发略蓬松,中等光泽,进食量减少,一般情况无恶化倾向。随时间进展,DM-Con组小鼠血糖进行性升高,而DM+Ex-4组血糖呈下降趋势,但仍明显高于Con组(P < 0.05)。到第16周时,较DM-Con组,DM+Ex-4组血糖显著性下降(P < 0.05),进食量显著减少(P < 0.05),体质量降低,但差异无统计学意义(表 1,图 2)。
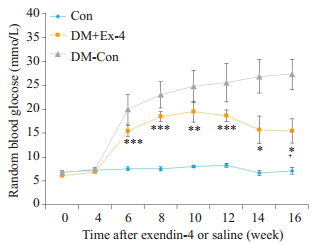
|
图 2 各组小鼠血糖比较 Figure 2 Changes of random blood glucose in the 3 groups (Means ± SE, n=6-8).*P < 0.05, **P < 0.01, ***P < 0.001 vs Con; +P < 0.05 vs DM-Con. |
小鼠宰杀后收集血清测血脂浓度,DM-Con组血清甘油三酯(TG)及低密度脂蛋白胆固醇(LDL-c)均显著高于Con组(P < 0.01)。Exendin-4干预后,糖尿病小鼠血清TG明显下降(P < 0.05),但仍显著高于Con组(P < 0.01);然而,DM+Ex-4组血清LDL-c较DM-Con组无明显改善(表 2)
| 表 2 第16周各组小鼠生理指标的比较 Table 2 Comparison of physical parameters in the 3 groups at week 16 (Mean±SEM, n=6-8) |
光镜下观察可见,与Con组相比,DM-Con组心机纤维排列明显紊乱,心肌肥大,间质可见纤维化;Exendin-4干预组(DM+Ex-4)心肌纤维排列明显改善,心肌肥大减轻,间质有少量疏松结缔组织(图 3)。
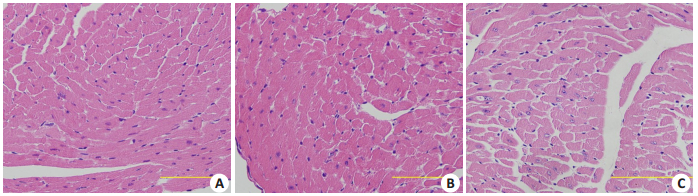
|
图 3 各组心肌组织HE染色 Figure 3 HE staining of myocardial tissue in the 3 groups (Original magnification: ×400; scale bar: 10 µm).A: Con; B: Con+ Ex-4; C: DM-Con. |
心房钠尿肽(ANP)、脑钠尿肽(BNP)是心房压力负荷的重要指标,能够有效评估心脏功能。转换生长因子β1(TGFβ1)是心肌纤维化的标志物。RT-PCR结果显示,DM-Con组ANP、BNP、TGFβ1表达较Con组显著升高,而exendin-4干预后,ANP、BNP、TGFβ1 mRNA水平明显降低(P < 0.05,图 4)。
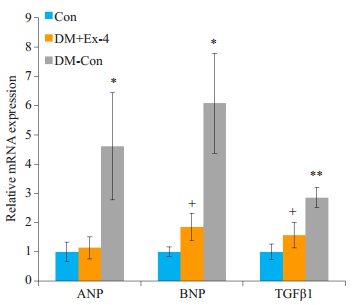
|
图 4 各组小鼠心肌中ANP、BNP、TGFβ1的表达情况 Figure 4 Myocardial expression of ANP, BNP and TGFβ1 in the 3 groups (Means±SE, n=6).*P < 0.05, **P < 0.01 vs Con; +P < 0.05 vs DM-Con. |
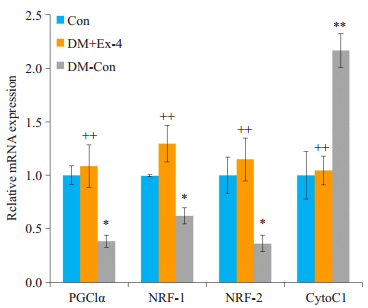
线粒体功能障碍参与糖尿病心肌病的发生发展,过氧化物酶增殖物激活受体γ共激活因子1α(PGC1α)和核呼吸因子(NRF)是调节线粒体合成和功能的重要转录因子,细胞色素C(CytoC)是线粒体功能的重要标志物。RT-PCR结果表明,糖尿病小鼠心肌PGC1α、NRF-1、NRF-2的转录水平较正常小鼠明显下降(P < 0.05),Exendin-4干预后,PGC1α、NRF-1表达显著升高(P < 0.01);而与DM- Con组相比,DM + Ex- 4组CytoC1 mRNA水平明显降低(P < 0.01,图 5)。
|
图 5 各组小鼠心肌中线粒体功能相关基因表达情况 Figure 5 Myocardial expression of mitochondrial function-related genes in the 3 groups (Means±SE, n= 6).*P < 0.05, **P < 0.01 vs Con; ++P < 0.01 vs DM-Con. |
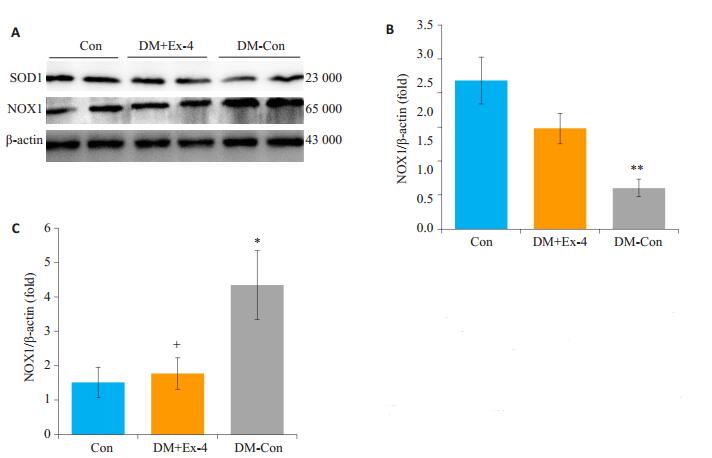
NADPH氧化酶(NOXs)主要通过质膜传递电子产生活性氧[17],超氧化物歧化酶(SOD)在保护细胞免受氧自由基损害中发挥着重要作用[18]。Western blot法检测各组NOX1、SOD1蛋白表达水平,结果发现,DM-Con组NOX1表达较Con组显著升高(P < 0.05),DM+Ex-4组NOX1表达较DM-Con组明显降低(P < 0.05)。与之相反,DM-Con组SOD1表达较Con组显著降低(P < 0.01),Exendin-4干预后,SOD1表达较DM-Con组明显升高(P < 0.05,图 6)。
|
图 6 各组小鼠心肌中SOD1、NOX1蛋白表达情况 Figure 6 Myocardial expression of SOD1 and NOX1 in the 3 groups (Means ± SE, n=6).A: Western blotting showing SOD1, Nox1 and β-actin expressions.B, C: Densitometric analysis of Western results.*P < 0.05, **P < 0.01 vs Con; +P < 0.05 vs DM-Con. |
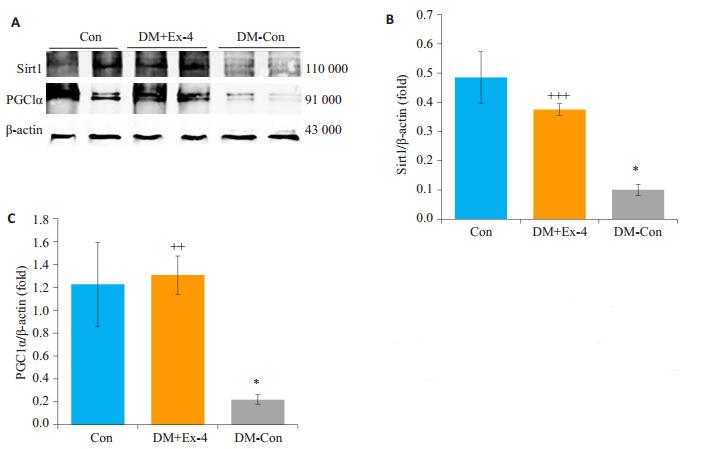
Western blot法检测各组Sirt1/PGC1α蛋白表达水平,结果发现,与Con比较,DM-Con组小鼠心肌组织中的Sirt1/PGC1α蛋白表达明显降低(P < 0.05);Exendin-4干预后,糖尿病小鼠心肌Sirt1/PGC1α蛋白表达较DM-Con组显著升高(P < 0.01,图 7)。
|
图 7 各组小鼠心肌中Sirt1、PGC1α蛋白表达情况 Figure 7 Myocardial expression of Sirt1 and PGC1α in the 3 groups (Means ± SE, n=6).A: Western blotting for SIRT1, PGC1α and β-actin in hearts.B, C: Densitometric analysis of western results.*P < 0.05 vs Con; ++P < 0.01, +++P < 0.001 vs DM-Con. |
本研究采用高脂饮食联合腹腔注射单剂量STZ建立2型糖尿病小鼠模型,应用高脂饲料使小鼠产生一定程度的胰岛素抵抗,再用STZ破坏部分胰岛β细胞,导致胰岛素分泌不足,这种传统方法成膜率高且稳定,与临床2型糖尿病也比较符合,适用于糖尿病及其并发症发病机制及药物疗效的评估[19]。本文结果显示实验第8周时,大部分小鼠(约80%)出现多饮、多食、多尿糖尿病症状,血糖大于13.9 mmol/L,考虑2型糖尿病模型造模成功。
越来越多证据证实GLP-1在调节糖脂代谢以及保护心血管方面有着重要作用[20-21]。Yi等[22]发现Exendin- 4通过抑制晚期糖基化终产物受体抑制高糖诱导的心肌细胞凋亡。Ravassa等在心肌细胞实验中发现GLP-1主要通过PI3K信号通路以及部分通过ERK1/2信号通路保护心肌细胞[23]。然而,关于GLP-1对糖尿病心肌病动物模型的影响的研究仍较少。本实验结果显示,糖尿病小鼠血糖、血脂较正常小鼠显著升高,心肌组织肥大及纤维化指标显著升高,心肌结构紊乱,心肌细胞肥大,符合糖尿病心肌病病变。Exendin-4干预8周后,心肌组织形态学损害明显改善,大部分心肌纤维排列较整齐,肥大心肌细胞减少;心肌组织BNP、TGFβ1表达明显降低;同时,血糖和血清甘油三酯显著降低。这与Monji等[13]应用exendin-4干预两种2型糖尿病心肌病模型(转基因KK小鼠和高脂饮食诱导的肥胖小鼠)的研究结果是一致的。以上结果均表明Exendin-4不仅改善全身糖脂代谢,而且能够延缓糖尿病小鼠的心肌损害。
DCM的病理机制目前仍未完全阐明,大量证据发现DCM与多种因素有关,包括心肌胰岛素信号传导受损、钙稳态受损、线粒体功能障碍、内质网应激、肾素-血管紧张素系统激活等[24],最终导致糖尿病心肌细胞肥大、间质纤维化。线粒体是细胞代谢的中心,参与了细胞的基本功能如ATP的产生、活性氧的产生和清除等,线粒体功能障碍和氧化应激在介导糖脂代谢紊乱以及糖尿病心肌功能失调方面至关重要[25-26]。沉默信息调节因子1(Sir1)是一种依赖于烟酰胺腺嘌呤二核苷酸(NAD+)的去乙酰化酶,能使多种蛋白质去乙酰化,从而调节细胞增殖、分化、凋亡以及能量代谢,在糖脂代谢调节和心血管系统中发挥重要作用。PGC1a是Sirt1下游的一个主要靶基因,负责调节参与心肌能量代谢、线粒体功能和心肌收缩力的基因[28]。当细胞能量储存减少,NAD+水平升高,激活Sirt1,进而去乙酰化PGC1a促进ATP的产生[29]。越来越多的研究表明,在生理及病理条件(如糖尿病,缺血再灌注等)下,Sirt1/PGC1a信号通路参与调节心肌代谢。Fang等发现白藜芦醇通过激活Sirt1/PGC1a改善线粒体功能,减轻氧化应激从而缓解糖尿病大鼠心肌损伤[30]。应用心脏特异性敲除Sirt1小鼠证实Sirt1在DCM进展中的保护作用[31]。此外,在糖尿病大鼠中GLP-1类似物利拉鲁肽通过激活AMPKSirt1通路抑制心脏脂肪变性,氧化应激和细胞凋亡[32]。本实验进一步观察到糖尿病小鼠心肌组织Sirt1/PGC1α蛋白表达明显下降,而Exendin-4干预能重新激活Sirt1/ PGC1α信号通路,继而上调下游蛋白如NRF-1、NRF-2,改善线粒体功能,改善氧化应激,从而减轻心肌损伤。
综上所述,Exendin-4能延缓糖尿病小鼠心肌损害的进展,其机制与Exendin-4改善全身糖脂代谢以及重新激活心肌组织Sirt1/PGC1α信号通路,改善线粒体功能,抑制氧化应激有关。这为临床防治糖尿病心肌病提供新思路。
| [1] |
Jia G, Whaley-Connell A, Sowers JR. Diabetic cardiomyopathy: a hyperglycaemia-and insulin-resistance-induced heart disease[J].
Diabetologia, 2018, 61(1): 21-8.
DOI: 10.1007/s00125-017-4390-4. |
| [2] |
Sung MM, Hamza SM, Dyck JR. Myocardial metabolism in diabetic cardiomyopathy: potential therapeutic targets[J].
Antioxid Redox Signal, 2015, 22(17): 1606-30.
DOI: 10.1089/ars.2015.6305. |
| [3] |
Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP[J].
Gastroenterology, 2007, 132(6): 2131-57.
DOI: 10.1053/j.gastro.2007.03.054. |
| [4] |
Nauck MA, Niedereichholz U, Ettler R, et al. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans[J].
Am J Physiol, 1997, 273(5 Pt 1): E981-8.
|
| [5] |
Turton MD, O'shea D, Gunn I, et al. Arole for glucagon-like peptide- 1 in the central regulation of feeding[J].
Nature, 1996, 379(6560): 69-72.
DOI: 10.1038/379069a0. |
| [6] |
Okerson T, Chilton RJ. The cardiovascular effects of GLP-1 receptor agonists[J].
Cardiovasc Ther, 2012, 30(3): e146-55.
DOI: 10.1111/cdr.2012.30.issue-3. |
| [7] |
Khan MA, Deaton C, Rutter MK, et al. Incretins as a novel therapeutic strategy in patients with diabetes and heart failure[J].
Heart Fail Rev, 2013, 18(2): 141-8.
DOI: 10.1007/s10741-012-9318-y. |
| [8] |
Bose AK, Mocanu MM, Carr RD, et al. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury[J].
Diabetes, 2005, 54(1): 146-51.
DOI: 10.2337/diabetes.54.1.146. |
| [9] |
Arakawa M, Mita T, Azuma K, et al. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon- like peptide-1 receptor agonist, exendin-4[J].
Diabetes, 2010, 59(4): 1030-7.
DOI: 10.2337/db09-1694. |
| [10] |
Bullock BP, Heller RS, Habener JF. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor[J].
Endocrinology, 1996, 137(7): 2968-78.
DOI: 10.1210/endo.137.7.8770921. |
| [11] |
Thorens B, Porret A, Bühler L, et al. Cloning and functional expression of the human islet GLP-1 receptor. Demonstration that exendin-4 is an agonist and exendin- (9-39) an antagonist of the receptor[J].
Diabetes, 1993, 42(11): 1678-82.
DOI: 10.2337/diab.42.11.1678. |
| [12] |
Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system[J].
Endocr Rev, 2012, 33(2): 187-215.
DOI: 10.1210/er.2011-1052. |
| [13] |
Monji A, Mitsui T, Bando YK, et al. Glucagon-like peptide-1 receptor activation reverses cardiac remodeling via normalizing cardiac steatosis and oxidative stress in type 2 diabetes[J].
Am J Physiol Heart Circ Physiol, 2013, 305(3): H295-304.
DOI: 10.1152/ajpheart.00990.2012. |
| [14] |
Kusakabe T, Tanioka H, Ebihara K, et al. Beneficial effects of leptin on glycaemic and lipid control in a mouse model of type 2 diabetes with increased adiposity induced by streptozotocin and a high-fat diet[J].
Diabetologia, 2009, 52(4): 675-83.
DOI: 10.1007/s00125-009-1258-2. |
| [15] |
Park CW, Kim HW, Ko SH, et al. Long-term treatment of glucagonlike peptide- 1 analog exendin-4 ameliorates diabetic nephropathy through improving metabolic anomalies in db/db mice[J].
J Am Soc Nephrol, 2007, 18(4): 1227-38.
DOI: 10.1681/ASN.2006070778. |
| [16] |
Kodera R, Shikata K, Kataoka HU, et al. Glucagon- like peptide-1 receptor agonist ameliorates renal injury through its antiinflammatory action without lowering blood glucose level in a rat model of type 1 diabetes[J].
Diabetologia, 2011, 54(4): 965-78.
DOI: 10.1007/s00125-010-2028-x. |
| [17] |
Hansen SS, Aasum E, Hafstad AD. The role of NADPH oxidases in diabetic cardiomyopathy[J].
Biochim Biophys Acta, 2018, 1864(5 Pt B): 1908-13.
|
| [18] |
Mccord JM, Edeas MA. SOD, oxidative stress and human pathologies: a brief history and a future vision[J].
Biomed Pharmacother, 2005, 59(4): 139-42.
DOI: 10.1016/j.biopha.2005.03.005. |
| [19] |
姜骊, 陈辉, 何勇, 等. 高脂饮食加低剂量链脲霉素建立小鼠2型糖尿病模型[J].
中国比较医学杂志, 2010(3): 35-8.
|
| [20] |
Saponaro F, Sonaglioni A, Rossi A, et al. Improved diastolic function in type 2 diabetes after a six month liraglutide treatment[J].
Diabetes Res Clin Pract, 2016, 118(8): 21-8.
|
| [21] |
Tian L, Jin T. The incretin hormone GLP-1 and mechanisms underlying its secretion[J].
J Diabetes, 2016, 8(6): 753-65.
DOI: 10.1111/1753-0407.12439. |
| [22] |
Yi B, Hu X, Wen Z, et al. Exendin-4, a glucagon-like peptide-1 receptor agonist, inhibits hyperglycemia-induced apoptosis in myocytes by suppressing receptor for advanced glycation end products expression[J].
Exp Ther Med, 2014, 8(4): 1185-90.
DOI: 10.3892/etm.2014.1873. |
| [23] |
Ravassa S, Zudaire A, Carr RD, et al. Antiapoptotic effects of GLP-1 in murine HL-1 cardiomyocytes[J].
Am J Physiol Heart Circ Physiol, 2011, 300(4): H1361-72.
DOI: 10.1152/ajpheart.00885.2010. |
| [24] |
Huynh K, Bernardo BC, Mcmullen JR, et al. Diabetic cardiomyopathy: mechanisms and new treatment strategies targeting antioxidant signaling pathways[J].
Pharmacol Ther, 2014, 142(3): 375-415.
DOI: 10.1016/j.pharmthera.2014.01.003. |
| [25] |
Aon MA, Foster DB. Diabetic cardiomyopathy and the role of mitochondrial dysfunction: novel insights, mechanisms, and therapeutic strategies[J].
Antioxid Redox Signal, 2015, 22(17): 1499-501.
DOI: 10.1089/ars.2015.6349. |
| [26] |
Khullar M, Al-Shudiefat AA, Ludke A, et al. Oxidative stress: a key contributor to diabetic cardiomyopathy[J].
Can J Physiol Pharmacol, 2010, 88(3): 233-40.
DOI: 10.1139/Y10-016. |
| [27] |
Kitada M, Koya D. SIRT1 in type 2 diabetes: mechanisms and therapeutic potential[J].
Diabetes Metab J, 2013, 37(5): 315-25.
DOI: 10.4093/dmj.2013.37.5.315. |
| [28] |
Koka S, Aluri HS, Xi L, et al. Chronic inhibition of phosphodiesterase 5 with tadalafil attenuates mitochondrial dysfunction in type 2 diabetic hearts: potential role of NO/SIRT1/PGC-1α signaling[J].
Am J Physiol Heart Circ Physiol, 2014, 306(11): H1558-68.
DOI: 10.1152/ajpheart.00865.2013. |
| [29] |
Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network[J].
Biochim Biophys Acta, 2011, 1813: 1269-78.
DOI: 10.1016/j.bbamcr.2010.09.019. |
| [30] |
Fang WJ, Wang CJ, He Y, et al. Resveratrol alleviates diabetic cardiomyopathy in rats by improving mitochondrial function through PGC-1α deacetylation[J].
Acta Pharmacol Sin, 2018, 39(1): 59-73.
DOI: 10.1038/aps.2017.50. |
| [31] |
Ma S, Feng J, Zhang R, et al. SIRT1 activation by resveratrol alleviates cardiac dysfunction via mitochondrial regulation in diabetic cardiomyopathy mice[J].
Oxid Med Cell Longev, 2017, 25(7): 4602715.
|
| [32] |
Inoue T, Inoguchi T, Sonoda N, et al. GLP- 1 analog liraglutide protects against cardiac steatosis, oxidative stress and apoptosis in streptozotocin- induced diabetic rats[J].
Atherosclerosis, 2015, 240(1): 250-9.
DOI: 10.1016/j.atherosclerosis.2015.03.026. |
 2018, Vol. 38
2018, Vol. 38

