2. Department of Chemistry, Bengbu Medical College, Bengbu 233030, China;
3. Department of Orthopedics, First Affiliated Hospital of Bengbu Medical College, Bengbu 233030, China
2. 蚌埠医学院 化学教研室,安徽 蚌埠 233030;
3. 第一附属医院骨科,安徽 蚌埠 233030
Osteosarcoma (OS) is the most common primary malignancy of the bone in children and adolescents aged 10 to 20 years.Local control surgery combined with post/ preoperative systemic multi-agent chemotherapy including cisplatin, epirubicin, etopside, methotrexate and cyclophosphamide[1-4] is currently a standard treatment for OS[2, 5]and leads to a 5-year survival rate of 60%-70% in the patients.Among the currently available drugs, however, either a single agent or a combined chemotherapy causes severe systemic toxicity, and new chemotherapeutic strategies with lower systemic toxicity are urgently needed for OS treatment.
MicroRNAs (miRNAs) are a class of non-coding RNAs 20 to 25 nucleotides in length.In many cellular, they regulate gene expressions through binding to partially complementary sequences of the target mRNAs processes [6-8], including virus defense, hematopoiesis, organogenesis, lipid metabolism as well as cell differentiation, proliferation and apoptosis [9].Currently increasing attention has been given to the relationship between miRNAs and cancers, and miRNAs have been found to promote or suppress tumor cell apoptosis, indicating their important roles in the occurrence and progression of tumors[10-13].
MiRNA-218 belongs to the SLIT2/SLIT3 gene family and usually functions as a tumor suppressor[10-13].Accumulating evidence suggests that miRNA-218 is frequently down-regulated in various cancers such as colorectal cancer [14], breast cancer [15], and clear cell renal cell carcinoma[16], but its role in OS remains unknown.In this study, using both clinical OS specimens and cultured OS cells, we aimed to investigate the role of miRNA-218 in the tumorigenesis and progression of OS and identify its potential target to explore the molecular mechanism underlying its tumor suppressing activity [17].The findings in this study may provide new insights into the molecular mechanisms of OS tumorigenesis and benefit the development of therapeutic strategies for OS treatment.
MATERIALS AND METHODS Clinical specimensThis study was conducted with the support of the First Affiliated Hospital of Bengbu Medical College.Primary osteosarcoma specimens were obtained from 42 male and 26 female patients diagnosed with OS, who were hospitalized in the First Affiliated Hospital of Bengbu Medical College between January, 2013 and June, 2015. All the patients underwent surgical resections of OS without any preoperative radiotherapy or chemotherapy. Both OS tissues and the adjacent normal tissues were stored at -80 ℃ immediately after collection for later miR-218 extraction.This study was approved by the Ethical Committee of the First Affiliated Hospital of Bengbu Medical College and written informed consent was obtained from each patient on a voluntary basis prior to sample collection.
Cell cultureOsteosarcoma Saos-2 cells were purchased from the Type Culture Collection of the Chinese Academy of Science (Shanghai, China).The cells were maintained in alpha modified Eagle's medium (α-MEM, purchased from Gibco-BRL) supplemented with 10% fetal bovine serum (FBS, Gibco-BRL) at 37 ℃ in a humidified atmosphere with 5% CO2.
Cell transfectionOverexpression and knockdown of miR-218 in Saos-2 cells were achieved by transfecting the cells with 100 nmol/L miR-218 mimics and anti-miR-218 mimics, respectively, using Lipofectamine 2000 (Invitrogen Life Technologies) in 60-mm dishes with antibiotic-free medium when the cells had reached 30%-50% confluence[18].The sequences of miR-218 mimics, anti-miR- 218 mimics and the negative control are listed below: miR-218 mimics:5'UUGUGCUUGAUCUAACCAUGU3', anti-miR-218 mimics: 5'ACAUGGUUAGAUCAAGCAC AA3', negative control: 5'UUCUCCGAACGUGUCACGU UU3' (double stranded).
All these constructs were purchased from GenePharma (Suzhou, China).
CCK-8 assay and annexin V-FITC cell apoptosis detectionCell apoptosis was detected using Cell Counting Kit-8 (CCK-8) assay.Briefly, Saos-2 cells transfected with miR- 218 mimics or anti-miR-218 were seeded into 96-well plates at a density of 8×103 cells per well.At 6, 24, 36, and 48 h after transfection, 10 μL of CCK-8 solution (DojinDo, Tokyo, Japan) was added into each well and the cells were further incubated for 1 h at 37 ℃.The absorbance at 450 nm (A450) was measured using a microplate reader.The Saos-2 cells that were not transfected with miR-218 or anti-miR-218 mimics served as the control.
For further quantitative detection of cell apoptosis, miR-218-transfected Saos-2 cells were harvested 24 h after transfection, and washed 3 times with PBS after centrifugation at 1500 r/min for 10 min.The cells were then stained with Annexin V-FITC Apoptosis Detection Kit (Ebioscience, USA) according to manufacturer's instructions.The cell apoptotic rates were analyzed with flow cytometry (Coulter Cytoflex, Beckman, USA).We also observed the morphology of the apoptotic cells with fluorescence microscope (BX53, Olympus, Japan).All the experiments were done in triplicate.
RNA extraction and quantitative real-time PCR (qRTPCR)Total RNA were extracted from the clinical specimens using Trizol reagent (TaKaRa Biotechnology, Dalian, China).The extracted RNA was eluted in RNase-free water at -20 ℃ and the RNA concentration was measured using a NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific, DE, USA).Reverse transcription was carried out using an All-in-OneTM First-Strand cDNA Synthesis Kit (AORT-0050, GeneCopoeiaTM, USA) according to the manufacturer's instructions.qRT-PCR was run on StepOnePlusTM Real-Time PCR system (Thermo Fisher Scientic), and the reaction was carried out in a total volume of 50 μL containing 1 μL cDNA, 1 μL forward primers, 1 μL reverse primers, 25 μL 2 × RealStar Green Power Mixture and 22 μL RNase-free water.The PCR parameters were as follows: 95 ℃ for 10 min, followed by 40 cycles of 95 ℃ for 15 s and 60 ℃ for 1 min (GeneStar Biosolutions, Beijing, China).The reactions were performed in triplicate for each sample.
Western blottingThe transfected cells were lysed in the lysis buffer containing 2% sodium dodecyl sulfate with 2 mol/L urea, 10% glycerol, 10 mmol/L Tris-HCl (pH 6.8), 10 mmol/L dithiothreitol and 1 mmol/L phenylmenthylsulfonyl fluoride.The lysed cells were centrifuged at 12 000 × g for 15 min at 4 ℃.The total proteins were separated on 10% SDS-PAGE gels and then transferred to nitrocellulose (NC) membranes (Bio-Rad Laboratories), which were incubated with primary antibodies at 4 ℃ overnight and then with the secondary antibodies at room temperature for 1 h.The band signals were visualized by enhanced chemiluminescence (ECL Kit, Amersham Biosciences).The following primary antibodies were used: rabbit polyclonal anti-BMI-1 (2830, Cell Signaling Technology, USA, 1:1000 dilution), rabbit polyclonal anti-PARP (9542, Cell Signaling Technology, USA, 1: 1000 dilution), and mouse monoclonal anti-β-actin (A1978, Sigma-Aldrich, USA, 1: 1000 dilution) [19]. Horseradish peroxidase (HRP)-conjugated goat antirabbit IgG (H + L) antibody (7074, Cell Signaling Technology, USA, 1:2000 dilution) and HRP-conjugated horse anti-mouse IgG (H + L) antibody (7076, Cell Signaling Technology, USA, 1:2000 dilution) were used as the secondary antibodies.With β-actin as the reference, we detected the relative expression levels of C-PARP and BMI-1 proteins in all the groups.
Luciferase reporter assayPlasmids containing pEZX/BMI-1-3/-UTR or pEZX/BMI- 1-3'-UTR-mutant together with firefly and renilla luciferase, and miR-218 mimics or NC control, were transfected into Saos-2 cells using Lipofectamine 2000 (Invitrogen, USA).Twenty-four hours after transfection, firefly and renilla luciferase activities were measured using Dual-Luciferase Reporter Assay System (Promega). The results were normalized as described previously[20].
Statistical analysisStatistical analyses were performed using Prism (GraphPad).All the data are presented as Mean±SD.The differences between each group were analyzed using unpaired, two-tailed Student's t test or one-way ANOVA. A P value less than 0.05 was considered to indicate a statistically significant difference.Each experiment was performed in triplicate, and all the experiments were repeated at least 3 times.
RESULTS miR-218 is down-regulated in primary OS tissuesTo determine the role of miR-218 in the pathogenesis of OS, we first examined the expression levels of miR-218 in 68 pairs of OS tissue and adjacent tissue samples using qRT-PCR.No significant correlations were found between miR-218 expression levels and the clinicopathological features of the patients including age, gender, chemotherapy regimens and tumor stage. Compared with the paired adjacent tissues, the OS tissues showed a significantly lowered expression level of miR-218 by at least 8 folds (0.89±0.04 vs 0.11±0.01, t= 28.23, P < 0.001).
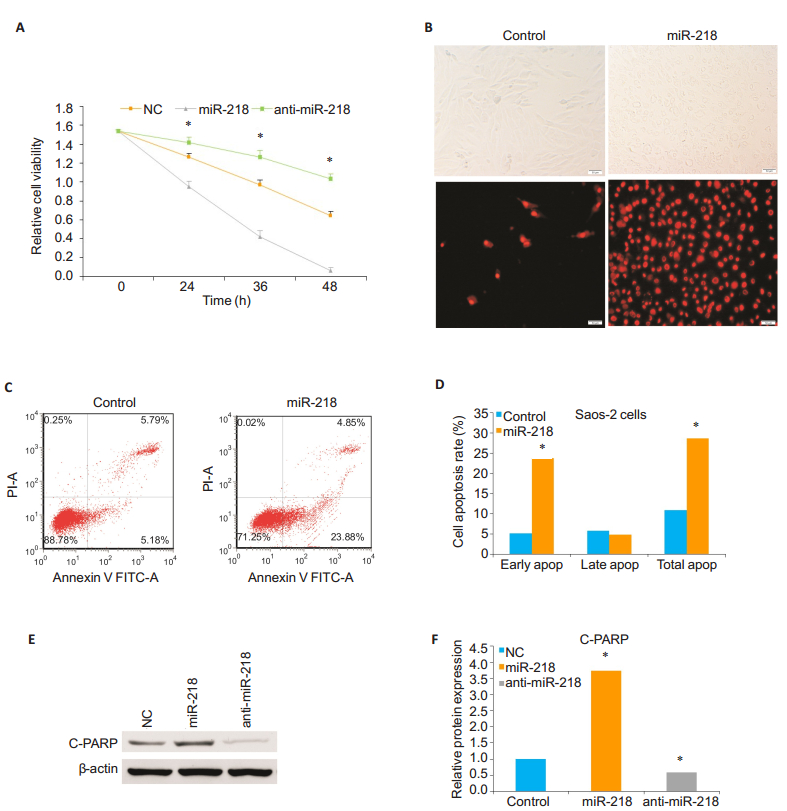
miR-218 induces apoptosis of Saos-2 cellsThe results of CCK-8 assay showed that compared with the cells transfected with anti-miR-218 mimics and the control cells, the cells transfected with miR-218 mimics for 24, 36, and 48 h exhibited significantly reduced cell viability, and transfection with anti-miR-218 mimics resulted in a significantly increased viability of the cells compared with the cells transfected with miR-218 mimics and the control cells (P < 0.05; Fig. 1A). Transfection of Saos-2 with miR-218 mimics caused reduced cell adhesion, whereas the cells transfected with anti-miR-218 mimics showed increased cell adhesion, suggesting the role of miR-218 in regulating cell apoptosis.We used flow cytometry and fluorescence microscope to observe the effect of miR-218 on cell apoptosis.Fig. 1B, 1C and 1D show that the number of apoptotic cells and cell apoptosis rate, especially early apoptosis rate, were significantly increased in miR-218- transfected cells as compared with the control cells (P < 0.05).To further confirm this, we performed Western blotting to detect the expression level of C-PARP, a marker of cell apoptosis, in Saos-2 cells transfected with miR-218 mimics or anti-miR-218 mimics.We found that the expression level of C-PARP was obviously increased in miR-218 group compared to that in the anti-miR-218 mimics group and control group (P < 0.05; Fig. 1E and 1F). All of these observations demonstrate that miR-218 overexpression promotes OS cell apoptosis while miR- 218 knockdown prevents OS cells from apoptosis.
|
Figure 1 Up- regulation of miR- 218 promotes osteosarcoma Saos- 2 cell apoptosis.A: Cell count of adhering living Saos- 2 cells transfected with miR-218 mimics or anti-miR-218 mimics and control Saos-2 cells, measured by CCK-8 at 24, 36 and 48 h; B: Morphological observation (phase- contrast microscopy, upper panel) and fluorescence microscopy (lower panel) of apoptotic cells 24 h after miR- 218 transfection with annexin V- FITC staining (original magnification: × 200, scale bar=50 μm); C: Flow cytometric analysis of apoptosis of Saos-2 cells and control cells 24 h after miR-218 transfection; D: Comparison of early, late and total apoptosis rates of Saos-2 cells between miR-218 transfected and non-transfected (control) groups; E, F: Western blotting and quantitative analysis of expression level of C-PARP protein relative to the control level in Saos-2 cells 24 h after transfection with miR-218 mimics or anti-miR-218 mimics.β-actin was used as a loading control.The data are presented as Mean±SD from 3 independent experiments (*Ρ < 0.05). |
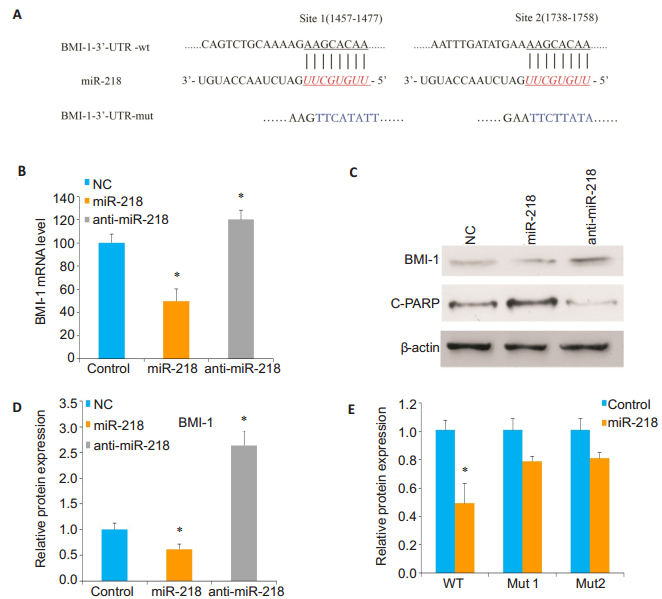
To identify the potential functional targets of miR-218 in OS cells, we used luciferase activity assay computational algorithms and discovered two complementary sites for miR-218 in the 3' untranslated region (3'-UTR) of BMI-1 gene (Fig. 2A).To confirm whether BMI-1 is a direct target of miR-218, qRT-PCR and Western blotting were performed to detect changes in BMI-1 mRNA and protein levels in Sao-2 cells transfected with miR-218 mimics or anti-miR-218 mimics.Compared to the control cells, the cells with miR-218 overexpression showed significantly reduced BMI-1 expressions at both mRNA and protein levels, while miR-218 knockdown caused enhanced BMI- 1 expression in the cells (P < 0.05; Fig. 2B, 2C and 2D).
|
Figure 2 MiR-218 directly targets BMI-1 in osteosarcoma Saos-2 cells.A: Predicted miR-218 target sequences in 3'UTR of BMI-1 mRNA and mutant BMI-1 mRNA containing 8 mutated nucleotides (BMI-1 mut); B: qRT-PCR analysis of BMI-1 expression in Saos- 2 cells transfected with miR- 218 mimics or anti- miR-218 mimics compared with negative control cells (*Ρ < 0.05); C, D: Western blotting and quantitative analysis of the expression level of BMI-1 relative to the control level in the transfected cells; E: Luciferase activity in Saos-2 cells co-transfected with miR-218 mimics and luciferase reporters containing either predicted miRNA target site in BMI-1 3'UTR or its corresponding mutant form (*Ρ < 0.05). |
To further confirm that miR-218 regulates the posttranscriptional effects of BMI-1 gene on 3'-UTR, luciferase reporter assay containing the full-length 3'- UTR region of BMI-1 (site 1457-1477 and site 1738- 1758) was performed.The luciferase activity of BMI-1 in Luc-BMI-1-UTR-transfected cells was significantly reduced compared with that of cells transfected with mutant BMI-1 3'-UTR and negative control cells (P < 0.05; Fig. 2E).Taken together, these results indicate that miR-218 regulates the expression level of BMI- 1 in OS cells by directly targeting BMI-1 3'-UTR.
DISCUSSIONGrowing evidence has suggested that miRNAs contribute to cancer initiation, development and metastasis in different types of cancers.miR-218 has proved to serve as a tumor suppressor in various human cancers[21-23], and has been shown to target several oncogenic genes, such as Rictor, survivin and ROBO1 receptor [24].A recent report indicated that up-regulation of miR-218 could inhibit cancer proliferation, migration, and invasion[21]. These studies suggest that miR-218 is an important tumor-suppressive miRNA and can be a potentially revolutionary clinical therapeutic target for cancers.In this study we compared the expression levels of miR-218 in primary OS and adjacent tissues and found significant down-regulation of miR-218 in OS tissues.In cultured Saos-2 cells, we found that transfection with miR-218 resulted in significantly increased cell apoptosis and suppressed cell proliferation, suggesting the role of miR-218 as a tumor suppressor in OS.Previous studies have identified an array of possible direct targets of miR-218, including Rictor[25], ECOP [26], ROBO1[27], LASP1[28], and PXN[29].Our result from luciferase report assay confirmed that BMI-1 is also a direct target of miR-218 in OS cells. All these results suggest that miR-218 overexpression in Saos-2 cells can down- regulate the expression level of BMI-1, which results in suppressed proliferation of Sao-2 cells.
Our results suggest that miR-218 may serve as a valuable candidate for devising therapeutic strategies for controlling progression of OS by up- regulating miR-218 or down-regulating BMI-1.Further study is stilled needed to understand how miR-218 is tightly regulated and whether there are other functional downstream effectors under regulation by miR-218.
| [1] | Isakoff M, Meltzer P, Gorlick R. Osteosarcoma: current treatment and a collaborative pathway to success[J]. J Clin Oncol, 2015, 33(27): 3029-35. DOI: 10.1200/JCO.2014.59.4895. |
| [2] | Anderson ME. Update on survival in osteosarcoma[J]. Orthop Clin North Am, 2016, 47(1): 283-92. DOI: 10.1016/j.ocl.2015.08.022. |
| [3] | Marina N, Gebharddt M, Teot L, et al. Biology and therapeutic advances for pediatric osteosarcoma[J]. Oncologist, 2004, 9(4): 422-41. DOI: 10.1634/theoncologist.9-4-422. |
| [4] | Wang XL, Zhang P, Bai X, et al. Targeted inhibition of mTORC2 prevents osteosarcoma cell migration and promotes apoptosis[J]. Oncol Rep, 2014, 32(1): 382-88. DOI: 10.3892/or.2014.3182. |
| [5] | Shao JL, Li ZZ, Zhou ZG, et al. Ganoderic acid a suppresses proliferation and invasion and induces apoptosis in human osteosarcoma cells[J]. J South Med Univ, 2015, 35(5): 619-24. |
| [6] | Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function[J]. Cell, 2004, 116(2): 281-97. DOI: 10.1016/S0092-8674(04)00045-5. |
| [7] | Song QX, Yang Y, Bai X. miR-483-5p promotes invasion and metastasis of lung adenocarcinoma by targeting RhoGDI1 and ALCAM[J]. Cancer Res, 2014, 74(11): 3031-42. DOI: 10.1158/0008-5472.CAN-13-2193. |
| [8] | Yang M1, Liu R, Sheng J, et al. Differential expression profiles of microRNAs as potential biomarkers for the early diagnosis of esophageal squamous cell carcinoma[J]. Oncol Rep, 2013, 29(1): 169-76. DOI: 10.3892/or.2012.2105. |
| [9] | Su Z, Yang Z, Xu Y, et al. MicroRNAs in apoptosis, autophagy and necroptosis[J]. Oncotarget, 2015, 6(11): 8474-90. |
| [10] | Lu YF, Zhang L, Zhang JF, et al. MiR-218 mediates tumorigenesis and metastasis: perspectives and implications[J]. Exp Cell Res, 2015, 334(1): 173-82. DOI: 10.1016/j.yexcr.2015.03.027. |
| [11] | Alajez NM, Ito E, Liu FF, et al. MiR-218 suppresses nasopharyngeal cancer progression through downregulation of survivin and the SLIT2- ROBO1 pathway[J]. Cancer Res, 2011, 71(6): 2381-91. DOI: 10.1158/0008-5472.CAN-10-2754. |
| [12] | Dallol A, Maher ER, Latif F, et al. SLIT2 axon guidance molecule is frequently inactivated in colorectal cancer and suppresses growth of colorectal carcinoma cells[J]. Cancer Res, 2003, 63(5): 1054-58. |
| [13] | Dickinson RE, Morton D, Latif F, et al. Epigenetic inactivation of SLIT3 and SLIT1 genes in human cancers[J]. Br J Cancer, 2004, 91(12): 2071-8. DOI: 10.1038/sj.bjc.6602222. |
| [14] | Yu H, Gao G, Huang J, et al. Decreased expression of miR-218 is associated with poor prognosis in patients with colorectal cancer[J]. Int J Clin Exp Pathol, 2013, 6(12): 2904-11. |
| [15] | Volinia S, Galassoa M, Crocea CM, et al. Breast cancer signatures for invasiveness and prognosis defined by deep sequencing of microRNA[J]. PNAS, 2012, 109(8): 3024-9. DOI: 10.1073/pnas.1200010109. |
| [16] | White NM, Bao TT, Yousef GM, et al. miRNA profiling for clear cell renal cell carcinoma: biomarker discovery and identification of potential controls and consequences of miRNA dysregulation[J]. J Urol, 2011, 186(3): 1077-83. DOI: 10.1016/j.juro.2011.04.110. |
| [17] | Zhang T, Wang T, Zhou J, et al. Down-regulation of miR-320 associated with cancer progression and cell apoptosis via targeting Mcl-1 in cervical cancer[J]. Tumour Biol, 2016, 37(7): 8931-40. DOI: 10.1007/s13277-015-4771-6. |
| [18] | Dong R, Yu J, Mao C, et al. Restoration of microRNA218 increases cellular chemosensitivity to cervical cancer by inhibiting cellcycle progression[J]. Mol Med Rep, 2014, 10(6): 3289-95. DOI: 10.3892/mmr.2014.2622. |
| [19] | Zhu L, Qiang F, Wang Y, et al. Downregulation of ubiquitin-specific protease 14 (USP14) inhibits breast cancer cell proliferation and metastasis, but promotes apoptosis[J]. J Mol Histol, 2016, 47(1): 69-80. DOI: 10.1007/s10735-015-9650-3. |
| [20] | Tu Y, Jin W, Zhang Y, et al. MicroRNA-218 inhibits glioma invasion, migration, proliferation, and cancer stem-like cell self-renewal by targeting the polycomb group gene Bmi1[J]. Cancer Res, 2013, 73(19): 6046-55. DOI: 10.1158/0008-5472.CAN-13-0358. |
| [21] | Cheng Y, Tao J, Lu Q, et al. MicroRNA-218 inhibits bladder cancer cell proliferation, migration, and invasion by targeting BMI-1[J]. Tumour Biol, 2015, 36(10): 8015-23. DOI: 10.1007/s13277-015-3532-x. |
| [22] | Tie J, Zhao L, Fan D, et al. MiR-218 inhibits invasion and metastasis of gastric cancer by targeting the robo1 receptor[J]. PLoS Genetics, 2010, 6(3): e1000879. DOI: 10.1371/journal.pgen.1000879. |
| [23] | Uesugi A, Omura K, Inazawa J, et al. The tumor suppressive microRNA miR-218 targets the mTOR component rictor and inhibits AKT phosphorylation in oral cancer[J]. Cancer Res, 2011, 71(17): 5765-78. DOI: 10.1158/0008-5472.CAN-11-0368. |
| [24] | Yang M, Liu R, Yin L, et al. Epigenetic repression of miR-218 promotes esophageal carcinogenesis by targeting ROBO1[J]. Int J Mol Sci, 2015, 16(11): 27781-95. DOI: 10.3390/ijms161126062. |
| [25] | Chen D, Cabay RJ, Zhou X, et al. MicroRNA deregulations in head and neck squamous cell carcinomas[J]. J Oral Maxillofac Res, 2013, 4(1): e2. |
| [26] | Tatarano S, Chiyomaru T, Hidaka H, et al. miR-218 on the genomic loss region of chromosoeme 4p15.31 functions as a tumor suppressor in bladder cancer[J]. Int J Oncol, 2011, 39(1): 13-21. |
| [27] | Xia H, Yan Y, Jiang X, et al. MiR- 218 sensitizes glioma cells to apoptosis and inhibits tumorigenicity by regulating ECOP-mediated suppression of NF-kappaB activity[J]. Neuro Onco, 2013, 15(4): 413-22. DOI: 10.1093/neuonc/nos296. |
| [28] | Nishikawa R, Goto Y, Kinoshita T, et al. Tumor suppressive microRNA- 218 inhibits cancer cell migration and invasion via targeting of LASP1 in prostate cancer[J]. Cancer Sci, 2004, 105(7): 802-11. |
| [29] | Wu DW, Cheng YW, Lee H, et al. Paxillin predicts survival and relapse in non-small cell lung cancer by microRNA-218 targeting[J]. Cancer Res, 2010, 70(24): 10392-401. DOI: 10.1158/0008-5472.CAN-10-2341. |
 2018, Vol. 38
2018, Vol. 38

