2. 陕西省中医医院检验科,陕西 西安 710003;
3. 西安交通大学 基础医学院,陕西 西安 710061
2. Clinical Laboratory of Shanxi Traditional Chinese Medicine Hospital, Xi'an 710003, China;
3. School of Basic Medical Science, Xi'an Jiaotong University, Xi'an 710061, China
胰腺癌被称为“癌中之王”,5年生存率不足5%[1]。据美国癌症协会统计,2018年美国新发胰腺癌患者预计达55 440例,因其死亡人数达44 330例[2-3]。而到2030年胰腺癌预计将成为美国第2大癌症死亡原因[4];同期,我国国家癌症中心发布的最新数据也显示,在中国胰腺癌的发病率和癌症相关死亡率亦呈上升趋势[5]。故寻找新的胰腺癌相关标记物及准确的诊断模型迫在眉睫[6]。随着研究的不断深入,研究者发现壳多糖酶3样蛋白(l CHI3L1)与肝癌、脑胶质瘤、子宫内膜癌等恶性肿瘤具有相关性[7-9];在胰腺癌中亦存在异常表达[10]。近年来,有关胰腺癌血清学标志物诊断效能方面的研究较多,既有单项血清标志物的研究,也有多项血清标志物联合检测对胰腺癌诊断的研究[11-12],然而未见CHI3L1联合其它标志物检测在胰腺癌中诊断价值的研究。本研究在前期研究的基础上,首次初步探讨CHI3L1、CA199、高密度脂蛋白胆固醇(HDL-C)、低密度脂蛋白胆固醇(LDL-C)、补体3(C3)、补体4(C4)单独及联合检测在胰腺癌患者诊断中的应用价值。
1 资料和方法 1.1 研究对象收集2014年7月~2016年7月在西安交通大学第一附属医院就诊的胰腺癌患者血清70份,正常人血清31份,胰腺癌患者TNM分期参照《TNM及病理分期系统(AJCC第7版)》,胰腺癌组患者均经病理、实验室检查或影像学(超声、CT和核磁共振等)等手段确诊;健康对照组经临床体检,包括常规生化检查、超声波检查、心电图、胸片等排除有心脏、肝脏及肾脏等重要器官疾病的患者,且胰腺癌患者和正常对照者均无原发性脂代谢异常疾病。
1.2 方法全部受试者均于清晨空腹肘静脉采全血,分离血清。采用杭州普望生物技术有限公司提供的ELISA试剂盒检测血清中CHI3L1的水平、罗氏E170电化学发光分析仪及其配套试剂测定CA199、SIEMENS BNⅡ特种蛋白分析仪及其配套试剂测定C3和C4、LABOSPECT-008全自动生化分析仪测定HDL-C、LDL-C(试剂由日本和光纯药工业株式会社提供);各指标均严格按照仪器的标准操作规程进行检测。
1.3 统计学分析数据资料用SPSS18.0统计软件分析:计量资料以中位数和四分位数间距[M(P25-P75)]表示;正态分布资料,两组间比较采用t检验,多组间均数比较采用单因素方差分析;非正态分布资料采用非参数检验;利用SAS9.2软件绘制胰腺癌各检测指标相对于健康人群的受试者工作特征曲线(ROC),比较曲线下面积(AUC)。双侧P<0.05为差异具有统计学意义。
2 结果 2.1 血清中CHI3L1的表达与临床病理参数收集70例胰腺癌患者血清,其中男性29例,女性41例,其它临床指标见表 1,利用ELISA方法检测血清中CHI3L1蛋白水平,统计学分析CHI3L1蛋白的表达与临床病理指标的关系,结果显示:CHI3L1蛋白阳性表达与患者是否接受抗肿瘤治疗具有明显相关性(P= 0.019),与性别、年龄、转移等其它临床病理特征无明显的相关性(P>0.05)。
| 表 1 胰腺癌患者血清中CHI3L1的表达与临床病理参数之间的关系 Table 1 Association between serum CHI3L1 level and the clinicopathological parameters of patients with pancreatic cancer |
为了进一步明确血清中CHI3L1以及其它血清学指标在健康和肿瘤患者中的差异,我们比较了31例正常人和70例胰腺癌患者血清中CHI3L1、CA199、C3、C4、HDL-C、LDL-C的含量,发现胰腺癌患者血清中CHI3L1、CA199、C3、C4水平均明显升高,而HDL-C、LDL-C水平明显降低,结果均具有统计学意义(P<0.05,表 2)。
| 表 2 31例正常人和70例胰腺癌患者血清各指标比较 Table 2 Comparison of serum biomarkers between healthy subject and patients with pancreatic cancer |
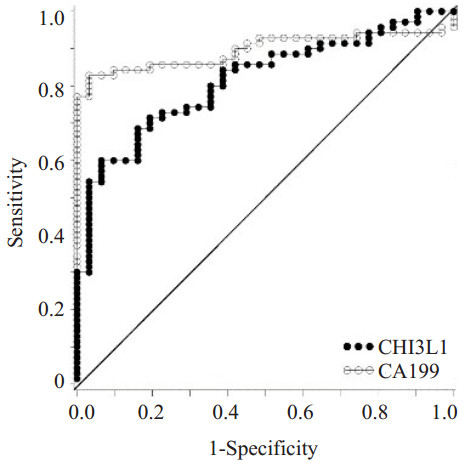
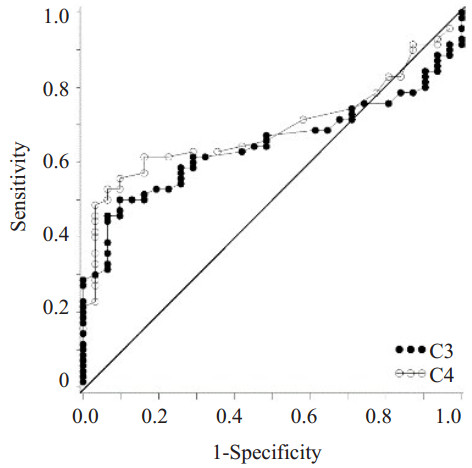
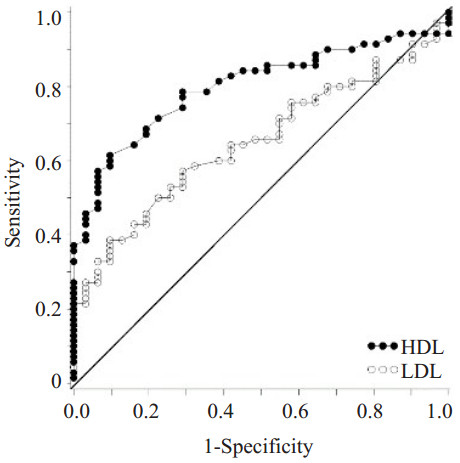
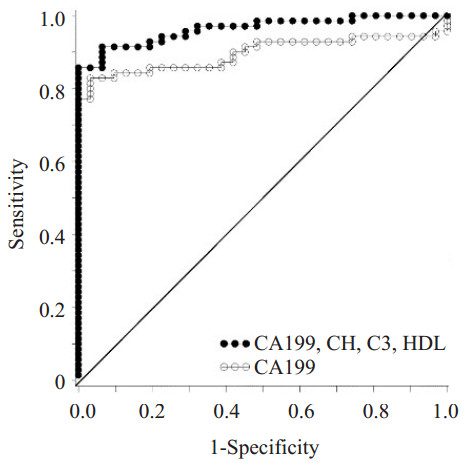
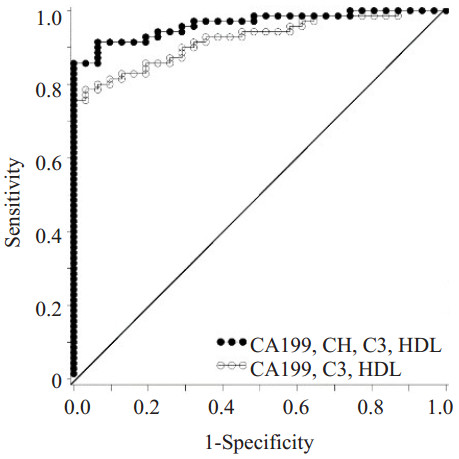
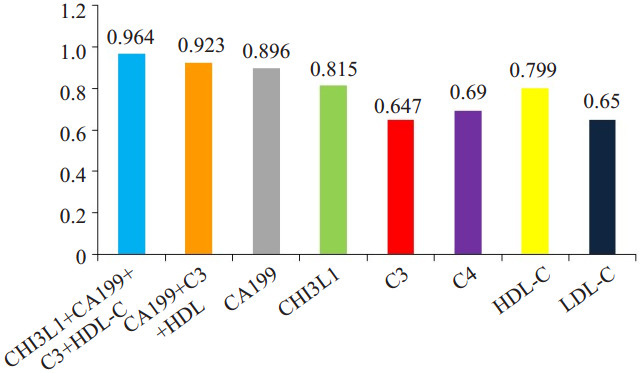
为了研究胰腺癌患者血清指标的诊断价值,我们分析了胰腺癌患者各检测指标的ROC曲线:在胰腺癌患者血清中CHI3L1、CA199、C3、C4、HDL-C、LDL-C水平AUC分别为0.815、0.896、0.647、0.690、0.799、0.650,而CHI3L1和CA199对胰腺癌具有较高的诊断价值;多指标联合分析发现:联合检测血清CHI3L1、CA199、C3、HDL-C(AUC=0.964)比各项指标单独检测及CA199、C3、HDL-C多因素联合检测(AUC=0.923)均具更高的诊断价值(P<0.05),此模型在胰腺癌患者中具有更高的诊断价值(图 1~6)。
|
图 1 胰腺癌血清中CHI3L1与CA199单独检测ROC曲线 Figure 1 ROC curves of CHI3L1 and CA199 in serum of pancreatic cancer patients (AUCCHI3L1=0.815, AUCCA199=0.896). |
|
图 2 胰腺癌血清中C3与C4单独检测ROC曲线 Figure 2 ROC curves of C3 and C4 in serum of pancreatic cancer patients (AUCC3=0.647, AUCC4=0.690). |
|
图 3 胰腺癌血清中HDL-C与LDL-C单独检测ROC曲线 Figure 3 ROC curves of HDL-C and LDL-C in serum of pancreatic cancer patients (AUCHDL-C= 0.799, AUCLDL-C=0.650). |
|
图 4 胰腺癌血清中CA199、CHI3L1、C3、HDLC指标联合检测与CA199单独检测ROC曲线 Figure 4 ROC curves of CA199 and combination of CA199, CHI3L1, C3 and HDL-C(AUCModel=0.964, AUCCA199=0.896, P=0.014). |
|
图 5 胰腺癌血清中CA199、CHI3L1、C3、HDLC联合检测与CA199、C3、HDL-C联合检测ROC曲线 Figure 5 ROC curves of combined detection of CA199, CHI3L1, C3, HDL-C and combination of CA199, C3 and HDL-C (AUCModel=0.964, AUCModel Without CHI3L1=0.923, P=0.025). |
|
图 6 胰腺癌患者各检测指标的AUC Figure 6 Comparison of the AUCs of different detection models. |
目前在胰腺癌的临床诊断指标中,CA199糖蛋白仍是众多肿瘤标志物中最有价值的指标[13]。研究[14-16]显示:血清中CA199的水平对于胰腺癌的临床诊断、预后评估和肿瘤转移具有较高的临床价值。然而CA199有其局限性,如它属于Lewis血型抗原,5%~10%的正常人缺少此基因,从而不表达CA199,因此这部分胰腺癌患者血清CA199水平即使到肿瘤晚期也可能不升高[17]。在本研究中,纳入的70例胰腺癌患者中亦有19例CA199水平在正常范围,占比为27%。CHI3L1是哺乳动物壳多糖酶家族的成员,又称为YKL-40。近年来学者们的研究[7-9]认为CHI3L1在肝细胞性肝癌、脑胶质瘤、子宫内膜癌等多种肿瘤中呈明显升高,且其水平高低与癌症的预后具有相关性[18-21];在胰腺癌患者血清中CHI3L1水平明显升高,且与TNM分期显著相关[10]。另外,近年的研究发现;肿瘤与补体系统间存在许多复杂的内在联系,在免疫应答中补体的作用可能不是简单的抑制或者促进效应,在肿瘤不同进展期有不同的免疫效应[22-23]。学者们[24]通过免疫组化及蛋白组学等方法证实:在胰腺癌组织中补体C3和C4b1呈高表达,且C4b1可能参与了胰腺癌进展。同时过度增殖的肿瘤细胞需摄取和消耗大量的营养物质,而人体储存能量和构成细胞膜的主要成分是脂质,因此,研究也提示:血脂代谢的异常在不同种类的癌症患者中具有不同特点[25-26]。研究表明[27]:消化系统肿瘤患者血清HDL-C水平与正常人相比显著降低,HDL-C水平与其分化程度、肿瘤大小及淋巴结转移有密切关系,HDL-C在有淋巴结转移的患者血清中显著降低,HDL-C也可能是作为保护因子而被消耗。本研究中,胰腺癌患者血清CA199、CHI3L1、C3/C4水平显著高于正常组(P<0.01),而HDL-C/LDLC水平显著低于正常组(P<0.01)。ROC曲线分析显示各指标对胰腺癌均具有一定的诊断价值(AUC分别为0.896、0.815、0.649/0.690、0.799/0.650),这与大部分的文献报道一致。在本研究中CHI3L1蛋白的表达与临床病理指标分析还发现,CHI3L1蛋白阳性表达与抗肿瘤治疗与否具有明显相关性(P=0.019),与性别、年龄等无明显的相关性。
本研究在前期研究的基础上,首次初步探讨CHI3L1与其他诊断模型指标联合检测的诊断效能。通过受试者工作特征曲线(ROC)分析发现:CA199、CHI3L1、C3、C4、HDL-C、LDL-C对胰腺癌患者均有一定的诊断价值,其中CA199的诊断价值最大,CHI3L1次之。多因素模型分析发现:在胰腺癌患者中联合检测CA199、C3和HDL-C优于CA199单独检测(AUC 0.923),具有较高的诊断价值,这与张宁[28]等的研究结果一致;本研究首次提出:联合检测血清中CHI3L1、CA199、C3和HDL-C,此诊断模型更优于联合CA199、C3和HDL-C检测(AUC 0.964),在胰腺癌患者中具有更高的诊断价值。此模型有望应用于胰腺癌的临床诊断,同时也为CHI3L1蛋白应用于胰腺癌诊断奠定了实验基础。
综上所述,CHI3L1表达水平在胰腺癌患者血清中呈高表达,CHI3L1蛋白有望成为胰腺癌新的诊断指标。通过单因素和多因素联合ROC曲线分析提示,联合检测血清中CHI3L1、CA199、C3和HDL-C,有望应用于胰腺癌的临床诊断。后期我们将加大样本量,严格控制纳入标准,以排除混杂偏倚,探讨CHI3L1在胰腺癌诊断中的应用价值。
| [1] |
Biankin AV, Waddell N, Kassahn KS, et al. Pancreatic Cancer genomes reveal aberrations in axon guidance pathway genes[J].
Nature, 2012, 491(7424): 399-405.
DOI: 10.1038/nature11547. |
| [2] |
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018[J].
CA Cancer J Clin, 2018, 68(1): 7-30.
DOI: 10.3322/caac.v68.1. |
| [3] |
Ashktorab H, Kupfer SS, Brim H, et al. Racial disparity in gastrointestinal cancer risk[J].
Gastroenterology, 2017, 153(4): 910-23.
DOI: 10.1053/j.gastro.2017.08.018. |
| [4] |
Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer[J].
Lancet, 2016, 388(10039): 73-85.
DOI: 10.1016/S0140-6736(16)00141-0. |
| [5] |
Chen W, Zheng R, Zhang S, et al. Cancer incidence and mortality in China in 2013: an analysis based on urbanization level[J].
Chin J Cancer Res, 2017, 29(1): 1-10.
DOI: 10.21147/j.issn.1000-9604.2017.01.01. |
| [6] |
Zhang Q, Chen S, Zeng L, et al. New developments in the early diagnosis of pancreatic cancer[J].
Expert Rev Gastroenterol Hepatol, 2017, 11(2): 149-56.
DOI: 10.1080/17474124.2017.1271323. |
| [7] |
Zhu CB, Chen LL, Tian JJ, et al. Elevated serum YKL-40 level predicts poor prognosis in hepatocellular carcinoma after surgery[J].
Ann Surg Oncol, 2012, 19(3): 817-25.
DOI: 10.1245/s10434-011-2026-3. |
| [8] |
Francescone RA, Scully S, Faibish M, et al. Role of YKL-40 in the angiogenesis, radioresistance, and progression of glioblastoma[J].
J Biol Chem, 2011, 286(17): 15332-43.
DOI: 10.1074/jbc.M110.212514. |
| [9] |
Diefenbach CS, Shah Z, Iasonos A, et al. Preoperative serum YKL-40 is a marker for detection and prognosis of endometrial cancer[J].
Gynecol Oncol, 2007, 104(2): 435-42.
DOI: 10.1016/j.ygyno.2006.08.028. |
| [10] |
Schultz NA, Christensen IJ, Werner J, et al. Diagnostic and prognostic impact of circulating YKL-40, IL-6, and CA 19.9 in patients with pancreatic cancer[J].
PLoS One, 2013, 8(6): e67059.
DOI: 10.1371/journal.pone.0067059. |
| [11] |
Kim J, Bamlet WR, Oberg AL, et al. Detection of early pancreatic ductal adenocarcinoma with thrombospondin-2 and CA19-9 blood markers[J].
Sci Transl Med, 2017, 9(398): 5583.
DOI: 10.1126/scitranslmed.aah5583. |
| [12] |
Mayerle J, Kalthoff H, Reszka R, et al. Metabolic biomarker signature to differentiate pancreatic ductal adenocarcinoma from chronic pancreatitis[J].
Gut, 2018, 67(1): 128-37.
DOI: 10.1136/gutjnl-2016-312432. |
| [13] |
Zhang Y, Jiang L, Song L. Meta-analysis of diagnostic value of serum carbohydrate antigen 199 in pancreatic cancer[J].
Minerva Med, 2016, 107(1): 62-9.
|
| [14] |
Chen Y, Gao SG, Chen JM, et al. Serum CA242, CA199, CA125, CEA, and TSGF are biomarkers for the efficacy and prognosis of cryoablation in pancreatic cancer patients[J].
Cell Biochem Biophys, 2014, 71(4): 1287-91.
|
| [15] |
Huang Z, Liu F. Diagnostic value of serum carbohydrate antigen 19-9 in pancreatic cancer: a meta-analysis[J].
Tumour Biol, 2014, 35(8): 7459-65.
|
| [16] |
Wang Z, Tian YP. Clinical value of serum tumor markers CA19-9, CA125 and CA72-4 in the diagnosis of pancreatic carcinoma[J].
Molecu Clin Oncol, 2014, 2(2): 265-8.
DOI: 10.3892/mco.2013.226. |
| [17] |
Luo G, Liu C, Guo M, et al. Potential biomarkers in lewis negative patients with pancreatic cancer[J].
Ann Surg, 2017, 265(4): 800-5.
DOI: 10.1097/SLA.0000000000001741. |
| [18] |
Johansen JS, Christensen IJ, Jørgensen LN, et al. Serum YKL-40 in risk assessment for colorectal cancer: a prospective study of 4, 496 subjects at risk of colorectal cancer[J].
Cancer Epidemiol Biomarkers Prev, 2015, 24(3): 621-6.
DOI: 10.1158/1055-9965.EPI-13-1281. |
| [19] |
Kjaergaard AD, Nordestgaard BG, Johansen JS, et al. Observational and genetic plasma YKL-40 and cancer in 96, 099 individuals from the general population[J].
Int J Cancer, 2015, 137(11): 2696-704.
DOI: 10.1002/ijc.v137.11. |
| [20] |
Tschirdewahn S, Reis H, Niedworok CA, et al. Prognostic effect of serum and tissue YKL-40 levels in bladder cancer[J].
Urolo Oncol, 2014, 32(5): 663-9.
DOI: 10.1016/j.urolonc.2014.02.004. |
| [21] |
Xu CH, Yu LK, Hao KK. Serum YKL-40 level is associated with the chemotherapy response and prognosis of patients with small cell lung cancer[J].
PLoS One, 2014, 9(5): e96384.
DOI: 10.1371/journal.pone.0096384. |
| [22] |
Bandini S, Curcio C, Macagno M, et al. Early onset and enhanced growth of autochthonous mammary carcinomas in C3-deficient Her2/neu transgenic mice[J].
Oncoimmunology, 2013, 2(9): e26137.
DOI: 10.4161/onci.26137. |
| [23] |
Janelle V, Langlois MP, Tarrab E, et al. Transient complement inhibition promotes a tumor-specific immune response through the implication of natural killer cells[J].
Cancer Immun Res, 2014, 2(3): 200-6.
DOI: 10.1158/2326-6066.CIR-13-0173. |
| [24] |
Chen J, Wu W, Zhen C, et al. Expression and clinical significance of complement C3, complement C4b1 and apolipoprotein E in pancreatic cancer[J].
Oncol Lett, 2013, 6(1): 43-8.
DOI: 10.3892/ol.2013.1326. |
| [25] |
Diemert A, Ortmeyer G, Hollwitz B, et al. The combination of intrauterine balloon tamponade and the B-Lynch procedure for the treatment of severe postpartum hemorrhage[J].
Am J Obstet Gynecol, 2012, 206(1): e1-4.
DOI: 10.1016/S0002-9378(11)02305-2. |
| [26] |
Chen DM, Li YZ, Hong Y, et al. The relationship of serum testosterone and its related index with the metabolism syndrome in women at perimenopausal or postmenopausal periods[J].
Zhonghua Fu Chan Ke Za Zhi, 2012, 47(2): 115-20.
|
| [27] |
Guo EQ, Chen LR, Xie QP, et al. Serum HDL-C as a potential biomarker for nodal stages in gastric cancer[J].
Ann Surg Oncol, 2007, 14(9): 2528-34.
DOI: 10.1245/s10434-007-9401-0. |
| [28] |
张宁, 王颖娴, 胡健, 等. 血清CA199、C3、C4及脂类代谢水平在胰腺癌临床诊断中的应用[J].
吉林大学学报:医学版, 2016, 42(2): 295-300.
|
 2018, Vol. 38
2018, Vol. 38

