2. 重庆医科大学附属儿童医院 儿科研究所干细胞实验室//儿童发育疾病研究所教育部重点实验室//儿童发育重大疾病国家国际科技合作基地//儿科学重庆市重点实验室,重庆 400014
2. Laboratory of Stem Cell Biology and Therapy, Children's Hospital of Chongqing Medical University, Ministry of Education Key Laboratory of Child Development and Disorders, China International Science and Technology Cooperation Base of Child Development and Critical Disorders, Chongqing Key Laboratory of Pediatrics, Chongqing 400014, China
随着医疗卫生体系的逐渐完善,人民的健康水平得到了极大提高,5岁以下儿童的死亡率已下降8.1‰[1],但出生缺陷仍是一个亟待解决的问题,胆汁淤积性肝硬化是新生儿外科中最为常见的疾病之一,尤其以胆道闭锁引起的为主,该病以进行性肝纤维化为特点,预后极差[2]。肝移植是目前彻底治愈该病的唯一方法,但受制于供体短缺、手术复杂、术后免疫排斥等原因,尚不能大规模应用。近年来,日益成熟的干细胞移植为疾病治疗提供了新的希望[3-5],肝脏干细胞来源于自身肝脏组织,具有向肝细胞和胆管细胞双向分化的潜力[6-8],是干细胞移植的理想细胞来源。然而,在胆汁淤积性肝硬化中,这一分化平衡受到哪些因素的调控,尚无报道,若能精确调节肝脏干细胞的分化,将为该病的干细胞移植治疗打下重要的理论基础。TNF-α作为一种常见的炎症因子,人们对它的认识已慢慢从免疫调节[9]转向组织器官发育[10-11];TGF-β1虽然在胚胎发育和组织、器官形成中有重要作用[12-13],但在肝脏疾病中更多的扮演着促纤维化的角色[14-15],它们对于肝脏干细胞分化的影响,目前所知甚少。本研究旨在检测TNF-α、TGF-β1在胆汁淤积性肝硬化中的变化,并深入详细探讨它们对肝脏干细胞分化平衡的影响。
1 材料和方法 1.1 主要材料与试剂雄性balb/c小鼠由重庆医科大学实验动物中心提供;小鼠胚胎肝脏干细胞株HP14-19由美国芝加哥大学医学分子肿瘤实验室合作分离鉴定;小鼠TNF-α、TGF-β1、ck19抗体(Abcam),ck18抗体(Proteintech),山羊抗兔IgG及山羊抗鼠IgG二抗,组化试剂盒(中杉金桥),Masson染色试剂盒(雷根生物);胎牛血清、DMEM高糖培养基、胰蛋白酶替代物(Gibco);TNF-α、TGF-β1细胞因子(Peprotech);过碘酸-希夫(periodicacid-schiff PAS)染色试剂盒(索莱宝);蛋白裂解液、BCA蛋白质浓度测定试剂盒(碧云天),ECL发光液(BIO-RAD);RNA提取试剂盒(凯基生物),RNA逆转录试剂盒及PCR试剂(Takara);PCR引物(华大基因)。
1.2 方法 1.2.1 动物模型的建立及检测选取54只6周龄balb/c小鼠,随机分为实验组(36只)和对照组(18只),术前禁食8 h,3.5%水合氯醛腹腔内注射麻醉,于腹正中线剑突下1 cm打开腹腔,暴露十二指肠上端,小心解剖分离出胆总管,实验组以6-0可吸收缝线结扎胆总管;对照组仅稍牵扯胆总管,不结扎,关闭腹腔,缝合消毒,以上操作均在无菌条件下进行。待小鼠苏醒后送回饲养笼,同等条件喂养,分别于术后不同时间点脱颈处死,观察肝脏大体结构改变,生理盐水灌注,取材,部分保存于液氮,部分保存于4%多聚甲醛,石蜡包埋行免疫组化、HE染色。
1.2.2 细胞培养与分组HP14-19细胞以含10%胎牛血清、100 U/mL青霉素、100 µg/mL链霉素的DMEM培养于37 ℃、5% CO2孵育箱中,待细胞贴壁生长至80%时,用胰蛋白酶消化传代。取对数生长期细胞接种于培养皿,待细胞贴壁后分别加入TNF-α、TGF-β1细胞因子,使其终浓度分别为10、20、40、80 ng/mL,同时设置空白对照组,本实验均重复3次或以上。
1.2.3 HE染色石蜡切片置于60 ℃烤箱2 h,二甲苯Ⅰ、Ⅱ各脱蜡、通透30 min,100%、95%、80%、75%梯度酒精各水化5 min,蒸馏水浸泡5 min;苏木素染色2 min,流水清洗1 min,盐酸酒精分化数秒,饱和碳酸锂返蓝,流水清洗1 min,伊红染色1 min,流水清洗,梯度酒精脱水,二甲苯透明、封片,显微镜拍照记录。
1.2.4 免疫组织化学染色石蜡切片按H&E染色步骤烘烤、脱蜡、水化;枸橼酸缓冲液微波煮沸修复30 min,冷却至室温;PBS摇床清洗3 min×3次;3%过氧化氢封闭10 min,PBS摇床清洗3 min×3次;分别滴加TNF-α、TGF-β1一抗4 ℃冰箱过夜;PBS摇床清洗3 min×3次,滴加试剂1,室温孵育20 min;PBS摇床清洗3 min×3次,滴加试剂2,室温孵育20 min,PBS摇床清洗3 min×3次。DAB(1:1000现配)显色。流水冲洗、苏木素复染、酒精梯度脱水,封片、拍照记录。
1.2.5 蛋白印迹检测相关蛋白表达从液氮中取出之前保存的肝脏组织,修剪至1 cm×1 cm×1 cm大小,置于2 mL EP管中,加入1 mL蛋白裂解液,电动匀桨器于冰上打碎,静止裂解10 min;培养的细胞分别在细胞因子诱导后第3、5、10天终止培养,按每6 cm dish加入100 µL蛋白裂解液处理;上述裂解液以14 000 r/min离心5 min,取上清。BCA法测蛋白浓度,SDS-PAGE电泳,PVDF膜100 v转膜,5%脱脂牛奶室温封闭1 h,TNF-α、TGF-β、ck18、ck19、GAPADH一抗4 ℃孵育过夜,二抗室温孵育1 h。ECL显影记录,Image-J软件分析条带灰度值。
1.2.6 Real-time PCR检测肝脏干细胞分化情况在细胞因子诱导后第3、5、10天终止培养,试剂盒提取各组细胞总RNA,逆转录合成cDNA。PCR扩增相关基因,引物序列见表 1,以GAPADH为内参标化模板,检测肝细胞标志物ck18和胆管细胞标志物ck19表达情况。
| 表 1 Real-time PCR引物序列 Table 1 Real-time PCR primer sequences |
将细胞以合适浓度接种于24孔板诱导,在相同时间点终止培养,进行PAS染色:倒掉培养基,PBS清洗3次,4%多聚甲醛固定20 min,PBS洗3次,0.5%高碘酸液处理15 min;流水冲洗5 min,滴加Schiff染液,染色15 min,流水清洗5 min,显微镜下观察拍照。
1.3 统计学分析计量资料以均数±标准差表示,SPSS13.0软件进行统计学分析,组间比较采用单因素方差分析;计数资料采用Fisher确切概率法,P < 0.05为差异有统计学意义。
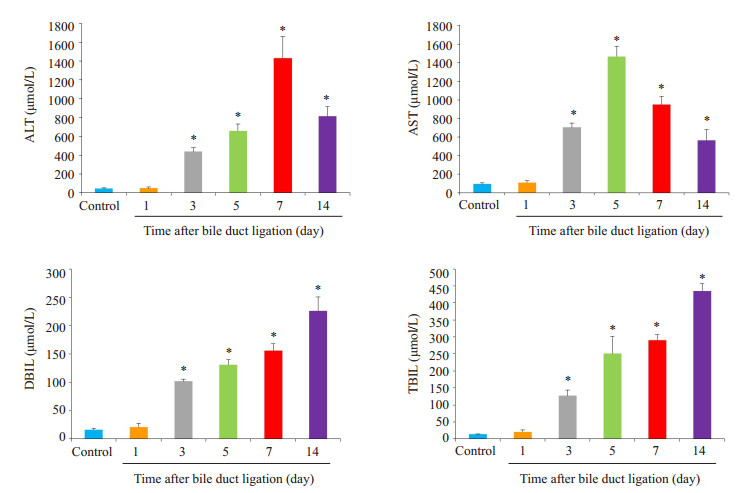
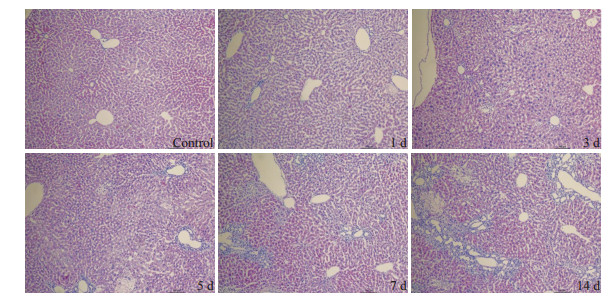
2 结果 2.1 动物模型的评价分别在小鼠胆总管结扎后第1、3、5、7、14天处死小鼠,检测血清TBIL、DBIL、AST、ALT变化(图 1,P < 0.05);HE染色观察大体形态及纤维增生(图 2);结果提示实验组各时间点的血清学指标不同程度高于对照组;HE染色可见随着造模时间延长,肝细胞发生不同程度的变性、坏死,间质可见炎症细胞浸润,胶原纤维增多,肝小叶结构紊乱。
|
图 1 胆总管结扎小鼠血清TBIL、DBIL、ALT、AST检测 Figure 1 Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), and direct Bilirubin (DBIL) in mice after bile duct ligation. *P < 0.05 vs control group. |
|
图 2 胆总管结扎小鼠肝脏HE染色 Figure 2 HE staining of the liver of mice after bile duct ligation (Original magnification: ×100). |
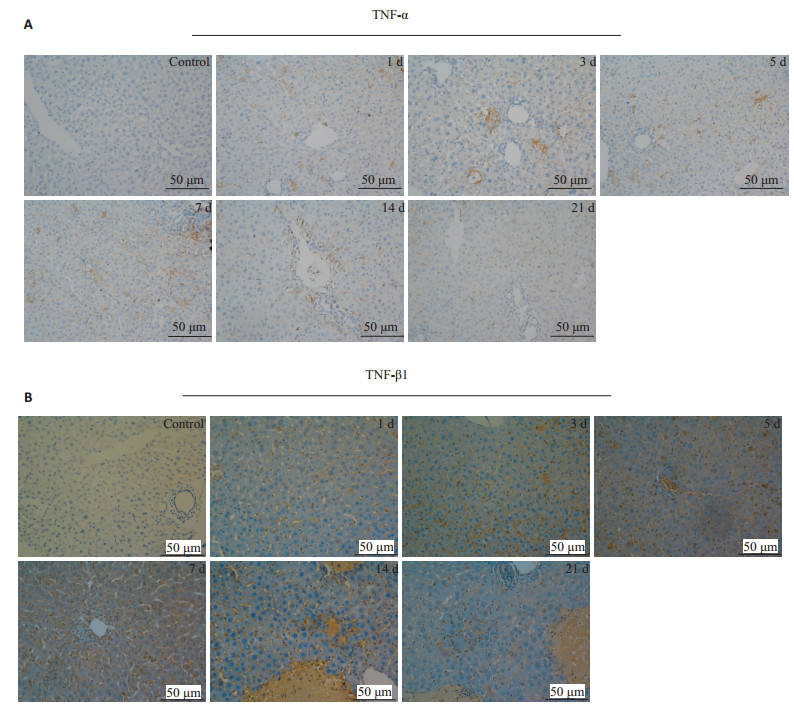
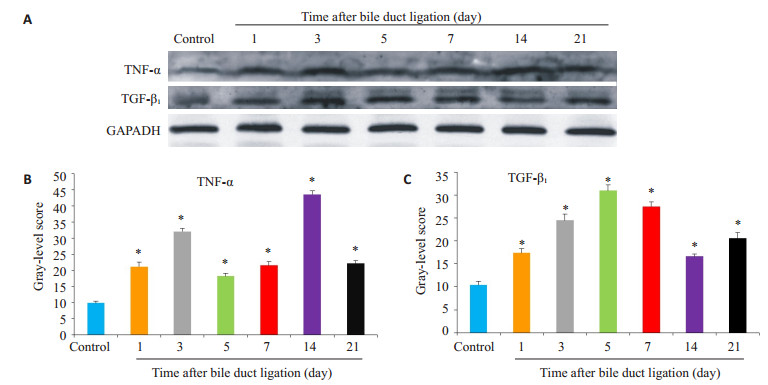
延长胆总管结扎的时间,分别在结扎后第1、3、5、7、14、21天处死小鼠,与假手术组一起行免疫组化(图 3)和蛋白印迹检测(图 4,P < 0.05);免疫组化提示实验组各时间点的TNF-α、TGF-β1表达均为阳性,且呈现出分泌性、弥散性表达,TNF-α以汇管区周围表达为主,而TGF-β1则主要在细胞间隙,窦周隙表达;蛋白印迹可见上述细胞因子在实验组的表达强于对照组(P < 0.05),且随着造模时间的延长,表现出“先上升、后下降”的趋势。
|
图 3 胆总管结扎小鼠肝脏免疫组织化学 Figure 3 Changes of expressions in TNF-α (A) and TGF-β1 (B) over time in the liver of mice after BDL (immunohistochemistry). |
|
图 4 胆总管结扎小鼠肝脏中细胞因子TNF-α、TGF-β1的表达 Figure 4 Expression of TNF-α and TGF-β1 in the liver of mice after bile duct ligation. A: Western blots of TNF-α and TGF-β1 in the liver; B, C: Quantitative analysis of results of Western blotting (*P < 0.05 vs control group) |
将诱导培养的HP14-19在第3天终止培养,提取各组细胞的蛋白、RNA,分别以蛋白印迹和Real-time PCR(图 5)检测ck18、ck19表达,结果显示在诱导3 d后,ck18和ck19的表达在实验组和对照组中暂未出现明显差别。
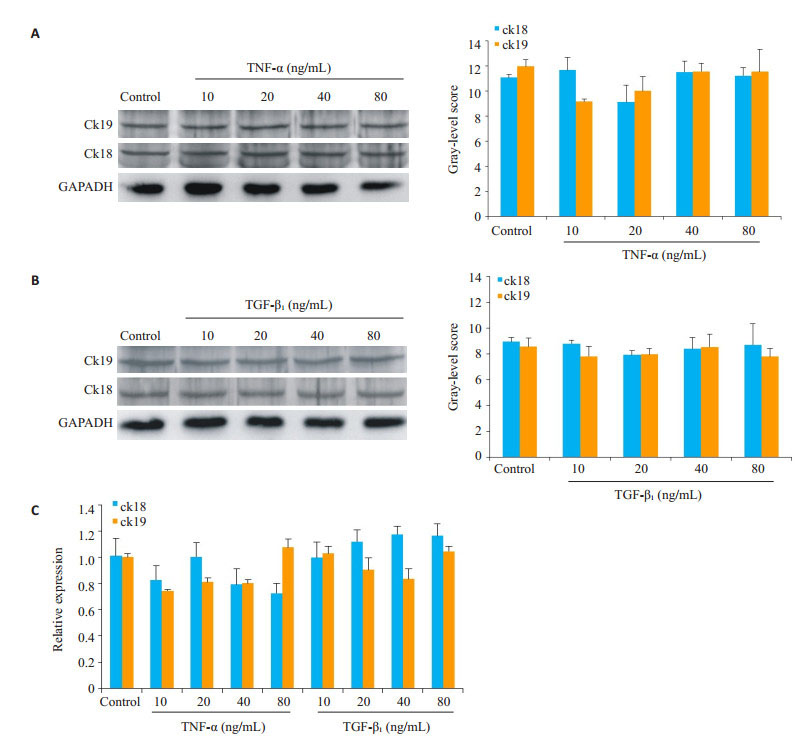
|
图 5 HP14-19在TNF-α、TGF-β1诱导3 d后ck18、ck19的表达 Figure 5 Expression of ck18 and ck19 in HP14-19 cells on the 3rd day of induction with TNF-α and TGF-β1. A: Western blots of ck18 and ck19 in TNF-α groups; B: Western blots of ck18 and ck19 in TGF-β1 groups; C: Real-time PCR for detecting expressions of ck18 and ck19 mRNAs. |
依照之前的方法,将诱导了5 d的HP14-19终止培养,再次以蛋白印迹和Real-time PCR检测ck18、ck19的表达(图 6,P < 0.05);PAS染色检测糖原累积情况(图 7)。结果显示:诱导5 d后,TNF-α、TGF-β1大部分浓度组的ck18、ck19均明显高于对照组,且在TNF-α20、40、80 ng/mL浓度组中,ck18表达高于ck19(P < 0.05),40 ng/mL浓度组的ck18表达最高(P < 0.05);而在TGF-β1中,则是20、40、80 ng/mL浓度组的ck19表达高于ck18(P < 0.05),20 ng/mL为ck19表达最高的浓度(P < 0.05);TNF-α各浓度组的PAS染色较对照组无明显变化,TGF-β1各浓度组的PAS染色则明显高于对照组。
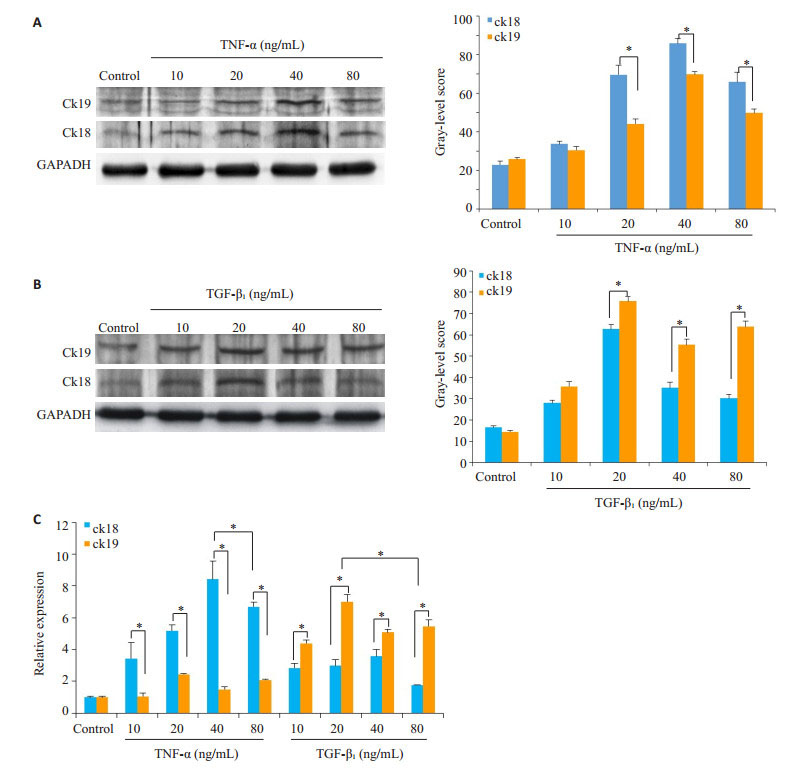
|
图 6 HP14-19在TNF-α、TGF-β1诱导5 d后ck18、ck19的表达 Figure 6 Expression of ck18 and ck19 in HP14-19 cells on the 5th day after induction with TNF-α and TGF-β1. A: Western blotting of the expressions of ck18 and ck19 in TNF-α groups (*P < 0.05 vs control group); B: Western blotting of the expressions of ck18 and ck19 in TGF-β1 groups (*P < 0.05 vs control group); C: Real-time PCR for detecting the expressions of ck18 and ck19 (*P < 0.05 vs control group). |
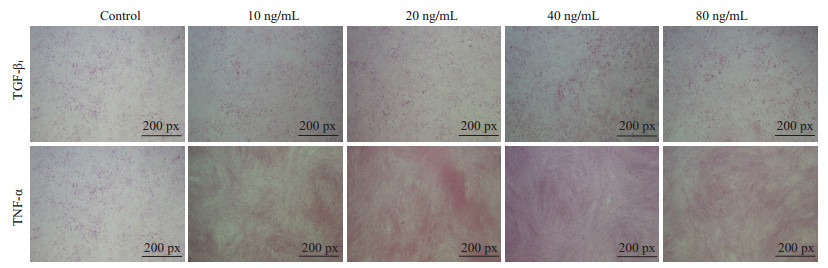
|
图 7 HP14-19在TNF-α、TGF-β1诱导5 d后PAS染色 Figure 7 PAS staining of HP14-19 cells on the 5th day after induction with TNF-α and TGF-β1. |
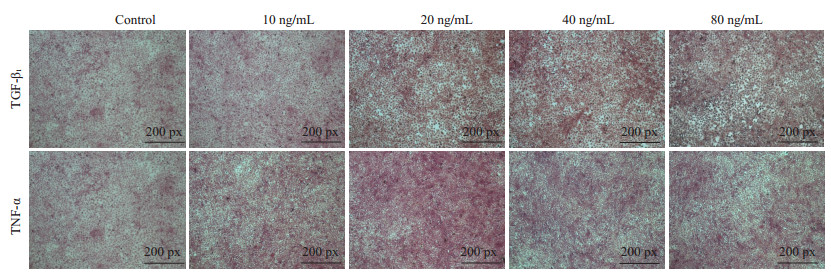
将HP14-19诱导分化的时间延长到10 d,再次以蛋白印迹、Realtime PCR、(图 8,P < 0.05)PAS染色评价其分化情况(图 9)。结果提示:当诱导时间延长到10 d时,TNF-α和TGF-β1在Real-time PCR、PAS染色和蛋白印迹结果上,其趋势与诱导5 d时相同,但Real-time PCR结果提示相较于诱导5 d时,TGF-β1各浓度在ck19的表达上已无明显差异。
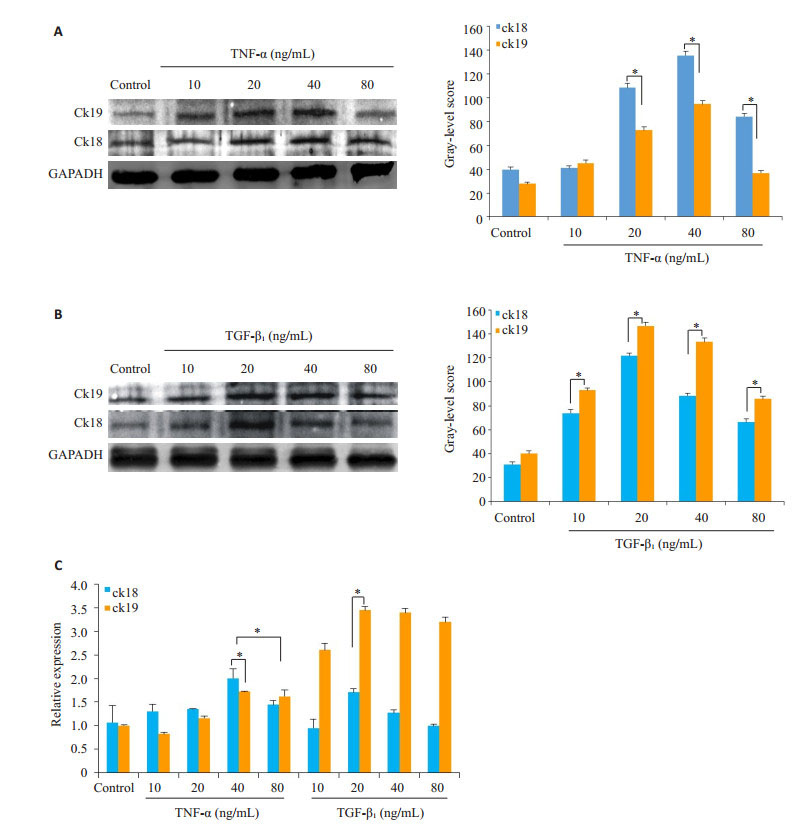
|
图 8 HP14-19在TNF-α、TGF-β1诱导10 d后ck18、ck19的表达 Figure 8 Expression of ck18 and ck19 in HP14-19 cells on day 10 of induction with TNF-α and TGF-β1. A: Western blotting of ck18 and ck19 in TNF-α groups (*P < 0.05 vs control group); B: Western blotting of ck18 and ck19 in TGF-β1 groups (*P < 0.05 vs control group); C: Real-time PCR of ck18 and ck19 mRNAs (*P < 0.05 vs control group). |
|
图 9 HP14-19在TNF-α、TGF-β1诱导10 d后PAS染色 Figure 9 PAS staining of HP14-19 cells after induction with TNF-α and TGF-β1 for 10 days. |
本研究先以小鼠胆总管结扎术模拟该疾病模型,术后血生化指标(TBIL、DBIL、ALT、AST)逐渐升高,提示肝细胞坏死,胆汁淤积、引流不畅;HE染色提示随着疾病的进展,胶原纤维增多,肝小叶紊乱。紧接着检测了疾病进展中不同时间点的TNF-α和TGF-β1,蛋白印迹发现它们均高于对照组,且在疾病进展中有一个表达高峰,免疫组化则提示它们在肝小叶中广泛分布,TNF-α作为炎症因子,主要分布在汇管区和炎症较重的地方,而TGF-β1则主要在细胞间隙和窦周隙分布。初步试验证明了TNF-α、TGF-β1在胆汁淤积性肝硬化疾病的肝脏组织微环境中高表达,但它们对肝脏干细胞分化有何作用,则需进一步研究。
继续将TNF-α、TGF-β1分浓度梯度刺激HP14-19,以肝细胞表面标志物ck18和胆管细胞表面标志物ck19的表达评价分化情况,通过3、5、10 d的诱导,发现TNF-α、TGF-β1对于肝脏干细胞的分化都有着促进作用,但前者更倾向于促进其向肝细胞分化,后者则倾向促进向胆管细胞分化,同时这种促分化作用有着剂量依赖性:TNF-α在40 ng/mL时,作用最强;而TGF-β1的最适浓度则是20 ng/mL;除此之外,在诱导5 d和10 d后的结果中还出现一个值得关注的现象:诱导10 d后的ck18、ck19在RNA水平上低于诱导5 d时,而在蛋白水平则是高于诱导5 d时,因为RNA的表达是一个短暂、瞬时的过程,而蛋白则是不断积累的,鉴于此,可以推断TNF-α、TGF-β1的促分化作用还有着时间依赖性,而这一效应在诱导第5天左右达到高峰。TNF-α和TGF-β1在促进肝脏干细胞分化上表现出如此大的差异可能与其各自涉及的信号通路相关:有研究称TNF-α与促进肝脏干细胞分化的Wnt/β-catenin信号[16-17]通路关系密切[18-19];TGF-β1则在多种模型中被发现能调控Jagged1/Notch的表达[20-21],而Notch通路和胆道形成有着直接而紧密的联系[22-24]。正是因为TNF-α和TGF-β1在Wnt和Notch信号通路上选择性的差异,最终影响了肝脏干细胞的分化。
另外,在PAS染色的结果中,还发现了一个有趣的现象:TGF-β1的PAS染色强于TNF-α,这提示TGF-β1有着更强的促进糖原合成能力,关于这一点,查阅文献后,解释如下:TGF-β1信号通路和GSK-3信号通路间存在着广泛的交互作用:在包括心、肾、肺、肝等多个器官的EMT模型中,TGF-β1能上调AKT/GSK-3β/β-catenin信号轴[25-26];而GSK-3又能促进TGF-β下游信号中的SMAD3的泛素化降解[27-28],正是因为GSK-3和TGF-β通路有着这样密切的相互作用,所以被TGF-β1诱导的肝脏干细胞能合成更多的糖原。
在对胆汁淤积性肝硬化的认识上,一直是肝炎引发纤维化,最终导致肝硬化。因此,抗炎和抗纤维化是常规治疗手段,但本研究发现TNF-α和TGF-β1除了能引发炎症和纤维化外,还能促进肝脏干细胞的分化,结合其他研究指出TNF-α参与了肝脏损伤后的自身修复[29-30],在今后的治疗中,对于轻微的肝脏炎症,是否进行抗炎治疗,或许有些启发;对于胆汁淤积性肝硬化而言,其根本病因在于胆汁引流不畅[1],本研究发现TGF-β1对于胆管细胞的形成有着促进作用,因此,适度的TGF-β1表达对疾病的治疗也很重要。遗憾的是本研究虽然探讨了TNF-α和TGF-β1对肝脏干细胞分化的作用,但在临床中,胆汁淤积性肝硬化患儿的TNF-α、TGF-β1表达如何,它们之间的比例是多少,还不清楚,而这对于肝脏干细胞的分化走向和疾病治疗都有着重要意义,在今后的研究中还需要进一步探讨。
综上所述,我们通过结扎胆总管成功模拟了胆汁淤积性肝硬化,发现TNF-α、TGF-β1在疾病进展中均被激活,且于肝小叶中弥散性表达;经过横向、纵向的比较,初步试验表明它们对肝脏干细胞的分化方向有着不同的作用:TNF-α主要促进肝脏干细胞向肝细胞分化;而TGF-β1则主要促进向胆管细胞分化,如能在今后的临床治疗中,根据这一平衡关系针对性的调节肝脏干细胞的分化,对疾病的治疗有着重大的意义。
| [1] |
国家统计局. 2016年《中国儿童发展纲要(2011-2020年)》统计监测报告[EB/OL]. http://www.stats.gov.cn/tjsj/zxfb/201710/t20171026_1546618.html, 2017-10-27.
|
| [2] |
Feldman AG, Mack CL. Biliary atresia: clinical lessons learned[J].
J Pediatr Gastroenterol Nutr, 2015, 61(2): 167-75.
DOI: 10.1097/MPG.0000000000000755. |
| [3] |
Yahng SA, Park SS, Jeon YW, et al. Successful outcomes of second hematopoietic stem cell transplantation with total nodal irradiation and ATG conditioning for graft failure in adult patients with severe aplastic anemia[J].
Bone Marrow Transplant, 2018, [Epub ahead of print].
|
| [4] |
Song Y, Zhou F, Song NX, et al. Impact on platelet recovery of recombinant human thrombopoietin in severe aplastic anemia patients with allogeneic hematopoietic stem cell transplantation[J].
Zhonghua Xue Ye Xue Za Zhi, 2018, 39(3): 207-11.
|
| [5] |
Niemeyer CM, Arico M, Basso G, et al. Chronic myelomonocytic leukemia in childhood: a retrospective analysis of 110 cases. European Working Group on Myelodysplastic Syndromes in Childhood (EWOG-MDS)[J].
Blood, 1997, 89(10): 3534-43.
|
| [6] |
Haque S, Haruna Y, Saito K, et al. Identification of bipotential progenitor cells in human liver regeneration[J].
Lab Invest, 1996, 75(5): 699-705.
|
| [7] |
Alison MR. Characterization of the differentiation capacity of ratderived hepatic stem cells[J].
Semin Liver Dis, 2003, 23(4): 325-36.
DOI: 10.1055/s-2004-815561. |
| [8] |
Williams MJ, Clouston AD, Forbes SJ. Links between hepatic fibrosis, ductular reaction, and progenitor cell expansion[J].
Gastroenterology, 2014, 146(2): 349-56.
DOI: 10.1053/j.gastro.2013.11.034. |
| [9] |
Park KM, Bowers WJ. Tumor necrosis factor-alpha mediated signaling in neuronal homeostasis and dysfunction[J].
Cell Signal, 2010, 22(7): 977-83.
DOI: 10.1016/j.cellsig.2010.01.010. |
| [10] |
Espín-Palazón R, Stachura DL, Campbell CA, et al. Proinflammatory signaling regulates hematopoietic stem cell emergence[J].
Cell, 2014, 159(5): 1070-85.
DOI: 10.1016/j.cell.2014.10.031. |
| [11] |
Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword[J].
Nat Rev Immunol, 2003, 3(9): 745-56.
DOI: 10.1038/nri1184. |
| [12] |
Wu MY, Hill CS. Tgf-beta superfamily signaling in embryonic development and homeostasis[J].
Dev Cell, 2009, 16(3): 329-43.
DOI: 10.1016/j.devcel.2009.02.012. |
| [13] |
Yang AT, Hu DD, Wang P, et al. TGF-β1 induces the dual regulation of hepatic progenitor cells with both anti-and proliver fibrosis[J].
Stem Cells Int, 2016, 2016: 1492694.
|
| [14] |
Park JH, Park B, Park KK. Suppression of hepatic Epithelial-toMesenchymal transition by melittin via blocking of TGFβ/Smad and MAPK-JNK signaling pathways[J].
Toxins (Basel), 2017, 9(4): E138.
DOI: 10.3390/toxins9040138. |
| [15] |
Kaimori A, Potter JJ, Choti M, et al. Histone deacetylase inhibition suppresses the transforming growth factor beta1-induced epithelialto-mesenchymal transition in hepatocytes[J].
Hepatology, 2010, 52(3): 1033-45.
DOI: 10.1002/hep.23765. |
| [16] |
Huch M, Dorrell C, Boj SF, et al. Wnt drives stem Cell-Mediated repair response after hepatic injury[J].
Hepatology, 2013, 58(5): 1847-50.
DOI: 10.1002/hep.26579. |
| [17] |
Heo J, Ahn EK, Jeong HG, et al. Transcriptional characterization of Wnt pathway during sequential hepatic differentiation of human embryonic stem cells and adipose tissue-derived stem cells[J].
Biochem Biophys Res Commun, 2013, 434(2): 235-40.
DOI: 10.1016/j.bbrc.2013.02.109. |
| [18] |
Qadir AS, Lee HL, Baek KH, et al. Msx2 is required for TNF-α-induced canonical Wnt signaling in 3T3-L1 preadipocytes[J].
Biochem Biophys Res Commun, 2011, 408(3): 399-404.
DOI: 10.1016/j.bbrc.2011.04.029. |
| [19] |
Luo X, Li HX, Liu RX, et al. Beta-catenin protein utilized by Tumour necrosis factor-alpha in porcine preadipocytes to suppress differentiation[J].
BMB Rep, 2009, 42(6): 338-43.
DOI: 10.5483/BMBRep.2009.42.6.338. |
| [20] |
Zavadil J, Cermak L, Soto-Nieves N, et al. Integration of TGF-beta/ Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition[J].
EMBO J, 2004, 23(5): 1155-65.
DOI: 10.1038/sj.emboj.7600069. |
| [21] |
Monteiro R, Pinheiro P, Joseph N, et al. Transforming growth factor β drives hemogenic endothelium programming and the transition to hematopoietic stem cells[J].
Dev Cell, 2016, 38(4): 358-70.
DOI: 10.1016/j.devcel.2016.06.024. |
| [22] |
Hofmann JJ, Zovein AC, Koh H, et al. Jagged1 in the portal vein mesenchyme regulates intrahepatic bile duct development: insights into Alagille syndrome[J].
Development, 2010, 137(23): 4061-72.
DOI: 10.1242/dev.052118. |
| [23] |
Boulter L, Govaere O, Bird TG, et al. Macrophage derived Wnt signalling opposes Notch signalling in a Numb mediated manner to specify HPC fate in chronic liver disease in human and mouse[J].
Nat Med, 2012, 18(4): 572-9.
DOI: 10.1038/nm.2667. |
| [24] |
Tanimizu N, Miyajima A. Notch signaling controls hepatoblast differentiation by altering the expression of liver-enriched transcription factors[J].
J Cell Sci, 2004, 117(Pt 15): 3165-74.
|
| [25] |
Guo X, Ramirez A, Waddell DS, et al. Axin and GSK3-control Smad3 protein stability and modulate TGF-signaling[J].
Genes Dev, 2008, 22(1): 106-20.
DOI: 10.1101/gad.1590908. |
| [26] |
Wang G, Matsuura I, He D, et al. Transforming growth factor-{beta}-inducible phosphorylation of Smad3[J].
J Biol Chem, 2009, 284(15): 9663-73.
DOI: 10.1074/jbc.M809281200. |
| [27] |
Millet C, Yamashita M, Heller M, et al. A negative feedback control of transforming growth factor-beta signaling by glycogen synthase kinase 3-mediated Smad3 linker phosphorylation at Ser-204[J].
J Biol Chem, 2009, 284(30): 19808-16.
DOI: 10.1074/jbc.M109.016667. |
| [28] |
Guo Y, Gupte M, Umbarkar P, et al. Entanglement of GSK-3β, β-catenin and TGF-β1 signaling network to regulate myocardial fibrosis[J].
J Mol Cell Cardiol, 2017, 110: 109-20.
DOI: 10.1016/j.yjmcc.2017.07.011. |
| [29] |
Webber EM, Bruix J, Pierce RH, et al. Tumor necrosis factor primes hepatocytes for DNA replication in the rat[J].
Hepatology, 1998, 28(5): 1226-34.
DOI: 10.1002/(ISSN)1527-3350. |
| [30] |
Yamada Y, Kirillova I, Peschon JJ, et al. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type Ⅰ tumor necrosis factor receptor[J].
Proc Natl Acad Sci USA, 1997, 94(4): 1441-6.
DOI: 10.1073/pnas.94.4.1441. |
 2018, Vol. 38
2018, Vol. 38

