食管癌是由食管鳞状上皮或腺上皮异常增生导致的恶性消化道肿瘤,我国是食管癌发病率和死亡率最高的国家[1]。目前,外科手术切除仍是治疗食管癌的基本措施,具体的手术方式多种多样,常用的开放式食管癌切除术(OE)有3种:包括左胸单切口手术、胸腹双切口手术和颈胸腹三切口手术[2]。最近随着国内外微创手术的发展,其在食管癌切除术中的应用也越来越广泛。与传统的OE相比,微创食管癌切除术(MIE)具有创伤小、疼痛少、安全性高、术后恢复快等优点[3]。
目前,食管癌的手术治疗已经非常成熟,但预后不良且5年生存率只有20% [4],其主要原因是由肿瘤的转移和复发引起的[5]。当前临床发现和诊断肿瘤的主要方法是肿瘤标志物检测和影像学检查[6]。肿瘤标志物检测有助于早期发现肿瘤,其缺点是特异性较差,易出现假阳性结果。影像学检查一般只能发现生长到一定大小的肿瘤,因此患者用这些方法检查出肿瘤时,往往已经失去了早期治疗的机会[7]。循环肿瘤细胞(CTCs)是指由肿瘤原发灶或转移灶进入血液循环的肿瘤细胞[8],在2007年CTCs被ASCO推荐为肿瘤标志物[9]。相关研究已明确可以在食管癌中检测到CTCs,并且动态监测CTCs对于评估食管癌患者的治疗效果有重要意义[10]。然而截至目前不同食管癌手术方式对CTCs水平是否存在影响罕有报道,且尚未有较全面的研究,本研究采用第二代CTCs检测技术-CanPatrolTM,对食管癌患者围手术期外周血CTCs进行动态监测,并对比分析MIE和OE术式在治疗食管癌患者中的作用及其对外周血CTCs水平的影响,为临床手术方式的选择及食管癌患者的预后判断提供参考。
1 资料和方法 1.1 临床资料随机选取2015年10月~2017年10月期间在本研究中心确诊的食管癌患者的临床资料,具体纳入标准为:(1)基于术前全身骨扫描和其他器官的影像学检查有手术指征且无转移性病变者;(2)通过术前常规检查对食管癌切除术可耐受者;(3)根据术前影像检查,肿瘤最长横径≤6 cm且无明显外侵的患者;(4)无明显因胸膜广泛粘连延长手术时间者;(5)术前未接受放疗和化疗的患者;(6)术后病理结果由第8版UICC/AJCC食管癌TNM分期系统进行病理分期[11]。排除标准:(1)不能耐受手术者;(2)手术资料不全者;(3)随访24周内患者死亡。最终,共73例食管癌患者被纳入MIE组(n=38)和OE组(n=35)。同时选择同期在本研究中心检查良性食管疾病患者(包括5例食管憩室症,3例贲门失弛缓症和2例食管良性肿瘤)和10例志愿者为对照组。本研究由我院伦理委员会批准,所有患者均签署书面知情同意书。
1.2 手术方式 1.2.1 MIE组Ivor-Lewis术式患者常规双腔气管内麻醉,腹部手术区消毒、铺巾。先平卧位腹腔镜游离胃,Hemolock钳闭胃左动脉后超声刀切断,保留胃网膜右血管弓,清扫腹腔及胃血管周围淋巴结后制作管状胃。然后取左侧卧位,胸腔镜下游离食管上至胸膜顶下至膈肌裂孔,清扫胸腔内淋巴结后上提管状胃至胸腔,经口置入Orvil钉砧行胸顶食管胃吻合。
Mc Keown术式:麻醉满意后,先左侧卧位,胸腔镜下游离食管上至胸膜顶下至膈肌裂孔并清扫胸腔内淋巴结,后平卧位,腹腔镜游离胃并进行腹腔淋巴结清扫,制作管状胃。于左侧颈部做一3~4 cm切口,游离颈段食管,上提管状胃至颈部行食管胃吻合。
1.2.2 OE组麻醉满意后,取右侧卧位。于左胸第6肋间后外侧做约20 cm切口进胸。游离食管,清扫胸腔内淋巴结,再经膈游离胃并清扫腹腔淋巴结,制作管状胃并提至胸腔,行主动脉弓上或弓下吻合。
1.3 检测方法本研究采用广州益善生物公司开发的将生物化学和细胞物理性质相结合的分离方法-CanPatrolTM技术检测食管癌患者外周血中的CTCs [12]。其原理为:首先裂解外周血中的红细胞,并利用CTCs与白细胞的大小差异通过纳米技术进行CTCs的分离和富集,然后通过多重mRNA原位分析方法对富集的CTCs进行特异性基因核酸定位,从而达到对CTCs进行分型和鉴定的目的[13]。
每例患者分别于术前、术中、术后进行血液样本采集。术前血液样本于手术前3 d采集;术中血液样本于肿瘤切除及淋巴结清扫完毕后采集;术后血液样本分别于术后3 d及术后2周采集。使用8号采血针和枸橼酸抗凝管采取8 mL外周血,血样采集后将采血管上下颠倒混匀10次,后将标本放置于冰袋中,24 h内送往广州益善生物公司实验室进行检测。CTCs阳性界定标准:食管癌患者外周血中检测到的CTCs数量≥2个/5 mL为阳性,而CTCs数量<2个/5 mL为阴性[14]。
1.4 统计方法使用SPSS22.0软件进行统计学分析。计量资料以均数±标准差表示。每一项资料均进行正态性检验及方差齐性检验。将正态分布的数据使用t检验和方差分析进行配对比较,而不成正态分布的数据则进行非参数秩和检验。计数资料以百分比和率表示,比较采用χ2或Fisher确切概率法检验。P<0.05认为差异具有统计学意义。
2 结果 2.1 两组食管癌患者一般资料比较和术前CTCs检测情况MIE组和OE组分别由38例和35例食管癌患者组成。两组间患者在年龄、性别、吸烟史、肿瘤部位、组织学类型及术后病理分期等方面差异均无统计学意义(P>0.05,表 1)。按照CTCs阳性界定标准,对照组10例食管良性疾病者和10例健康志愿者CTCs检测结果均为阴性。73例食管癌患者中,MIE组阳性率为55.3% (21/38),OE组阳性率为65.7% (23/35),两组之间CTCs阳性率比较差异无统计学意义(χ2=0.831,P=0.362,表 2)。
| 表 1 两组患者临床病理资料的比较 Table 1 Comparison of clinicopathological factors of the patients in the two groups[n (%) unless specified otherwise] |
| 表 2 MIE组和OE组患者术前CTCs阳性率的比较 Table 2 Comparison of circulating tumor cell-positive rate between MIE group and OE group[n (%)] |
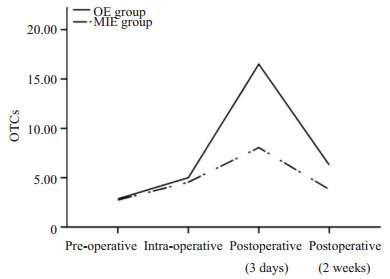
通过CanPatrolTM技术检测术前和术后CTCs水平。统计分析显示:在73例食管癌患者中,术中CTCs水平高于术前(z=-6.231,P=0.000),术后3 d CTCs水平高于术中(z=-7.381,P=0.000),差异均有统计学意义。MIE组和OE组CTCs水平在术前与术中比较差异无统计学意义(P>0.05),但MIE组术后(3 d)外周血CTCs水平显著低于OE组(z=-5.141,P=0.000,表 3)。CTCs增幅水平从术前至术后(3 d)在MIE组显著低于OE组(图 1),差异有统计学意义(z=-6.068,P=0.000)。
| 表 3 分别比较CTCs在MIE组和OE组各时点的水平 Table 3 Comparison of CTC levels at different stages in MIE group and OE group (Mean±SD) |
|
图 1 不同手术途径对围术期CTCs水平的影响 Figure 1 Effect of different surgical methods on CTC levels during the perioperative period. |
两组食管癌患者在手术时间、术中出血量及术后(2周)并发症的发生均有显著性差异(P<0.05,表 4)。MIE组术后(2周)总并发症发生率为28.9% (11/38),明显低于OE组的54.3%(19/35),差异有统计学意义(z=-2.277,P=0.023),术后2周再次检测每组患者的CTCs水平,在每组患者中,有严重并发症患者的CTCs水平显著高于手术结果良好的患者(P=0.001;P=0.005,表 5)。
| 表 4 两组患者术中、术后恢复情况及术后并发症的比较 Table 4 Intra-and postoperative complications in MIE group and OE group (Mean±SD) |
| 表 5 术后2周并发症的发生与CTCs水平的关系 Table 5 CTCs levels and occurrence of complications at 2 weeks after surgery (Mean±SD) |
目前,手术仍然是治疗食管癌最有效的措施,因为手术治疗可以将肿瘤整体切除并进行局部淋巴结清扫,使术后TNM分期更加精准,这对延长患者生存期、提高患者生活质量有重要作用[15-16]。随着科学技术的发展和医疗设施的不断完善,越来越多的研究人员开始探索MIE对食管癌的治疗效果[17],理论上,MIE术中腔镜的放大效果可以提供更加清晰的解剖层次,并可完成更加精细的操作。采用MIE治疗食管癌的患者手术切口小可以避免切断正常的肌肉组织,保留胸腔的固有结构,降低术后呼吸系统并发症的发生[18]。此外,MIE可以减少术中出血量,降低围术期死亡率,这些都充分说明MIE在治疗食管癌中的重要性[19-20]。
本文关于食管癌患者围术期不同阶段CTCs的研究表明,术中及术后CTCs的数量较术前有所增加,提示术中挤压肿瘤可促进肿瘤细胞机械性入血[21-22],术者应尽量避免挤压肿块,坚持术中无瘤原则。术后CTCs的数量显著高于术前及术中,提示术后早期机体免疫系统的紊乱可能会影响CTCs的动态变化。此外,食管癌患者术后,不仅其免疫系统紊乱,其自身内环境的改变导致其杀死肿瘤细胞的能力下降,由于CTCs的数量增加,使肿瘤细胞复发和转移发生的可能性显著增加[23]。对于大多数食管癌患者,尽管在其体内没有检测到多余的肿瘤细胞,但他们仍死于术后肿瘤的复发和转移,这些都可能与CTCs有关[24]。作为肿瘤复发和转移的新型诊断标准,CTCs可用于评估食管癌患者的预后效果[25-27]。
本研究对不同手术方式食管癌患者CTCs的变化研究结果显示,无论在MIE组还是OE组,术后有并发症患者的CTCs水平显著高于无并发症的患者,提示术后并发症的发生可能导致CTCs数量显著增加,加快肿瘤的复发和转移。此外,MIE组和OE组术中CTCs数量的变化无明显差异,但MIE组术后CTCs的水平显著低于OE组,而且MIE组术后有并发症患者的CTCs水平显著低于OE组,这些均提示MIE在控制CTCs方面比OE更有效[28]。有研究报告说明[29],OE术式简单容易、手术时间短、术中只需单一体位、手术器械较MIE便宜而且OE更广泛适用,MIE不能应用于晚期食管癌患者。因此,虽然MIE在控制CTCs方面更有效,但仍不能完全替代OE。然而,根据患者的实际情况,OE仍可是治疗食管癌的一个良好选择[30]。
综上所述,我们比较了MIE和OE对食管癌患者外周血CTCs的影响。结果表明,MIE在控制CTCs方面优于OE。然而,本研究在样本选择方面受到限制,因此得出的结论有一定偏差。我们还需进一步研究探讨CTCs与食管癌发病机制之间的联系、MIE较OE降低术后CTCs水平的机制及其与患者长期生存率之间的相关性。
| [1] |
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012[J].
Int J Cancer, 2015, 136(5): E359-86.
DOI: 10.1002/ijc.29210. |
| [2] |
Depypere L, Coosemans W, Nafteux P, et al. Video-assisted thoracoscopic surgery and open chest surgery in esophageal cancer treatment: present and future[J].
J Vis Surg, 2017, 3: 30.
DOI: 10.21037/jovs. |
| [3] |
Jeon HW, Sung SW. Minimally invasive ivor lewis esophagectomy for esophageal cancer[J].
J Vis Surg, 2016, 2: 165.
DOI: 10.21037/jovs. |
| [4] |
Jemal A, Bray F, Center MM, et al. Global cancer statistics[J].
CA Cancer J Clin, 2011, 61(2): 69-90.
DOI: 10.3322/caac.v61:2. |
| [5] |
Herszényi L, Pregun I, Tulassay Z. Diagnosis and recognition of early esophageal neoplasia[J].
Dig Dis, 2009, 27(1): 24-30.
|
| [6] |
李军. 食管鳞癌外周血循环肿瘤细胞检测及临床意义[D]. 广州: 南方医科大学, 2012.
http://cdmd.cnki.com.cn/Article/CDMD-90023-1013123657.htm
|
| [7] |
Saba NF. Esophagogastric junction and gastric adenocarcinoma: current challenges and future directions comment[J].
Oncol New York, 2014, 28(6): 520-1.
|
| [8] |
Boral D, Marchetti D. Liquid biopsy in prostate cancer: A case for comprehensive genomic characterization of circulation tumor cells[J].
Clin Chem, 2018, 64(2): 251-3.
DOI: 10.1373/clinchem.2017.283440. |
| [9] |
Wendel M, Bazhenova L, Boshuizen R, et al. Fluid biopsy for circulating tumor cell identification in patients with early-and latestage non-small cell lung cancer: a glimpse into lung cancer biology[J].
Phys Biol, 2012, 9(1): 016005.
DOI: 10.1088/1478-3967/9/1/016005. |
| [10] |
Sclafani F, Smyth E, Cunningham D, et al. A pilot study assessing the incidence and clinical significance of circulating tumor cells in esophagogastric cancers[J].
Clin Colorectal Cancer, 2014, 13(2): 94-9.
DOI: 10.1016/j.clcc.2013.11.003. |
| [11] |
Zhang D, Zheng Y, Wang Z, et al. Comparison of the 7th and proposed 8th editions of the AJCC/UICC TNM staging system for esophageal squamous cell carcinoma underwent radical surgery[J].
EJSO, 2017, 43(10): 1949-55.
DOI: 10.1016/j.ejso.2017.06.005. |
| [12] |
郭鑫, 陈群清. 液体活检技术在食管癌诊治中的研究进展[J].
广东医学, 2017(15): 2400-2.
|
| [13] |
郭柏棠, 刘新城, 黄毓, 等. 肝细胞癌患者外周血循环肿瘤细胞阳性提示预后不良[J].
南方医科大学学报, 2016, 36(8): 1134-9.
|
| [14] |
Gao Y, Zhu Y, Zhang Z, et al. Clinical significance of pancreatic circulating cells using combined negative enrichment and immunostaining-fluorescene in situ hybridization[J].
J Exp Clin Cancer Res, 2016, 35: 66.
DOI: 10.1186/s13046-016-0340-0. |
| [15] |
Jung J, Park SY, Park SJ, et al. Prognostic value of the neutrophilto-lymphocyte ratio for overall and disease-free survival in patients with surgically treated esophageal squamous cell carcinoma[J].
Tumour Biol, 2015, 37(6): 1-6.
|
| [16] |
Yanasoot A, Yolsuriyanwong K, Ruangsin S, et al. Costs and benefits of different methods of esophagectomy for esophageal cancer[J].
Asian Cardiovasc Thorac Ann, 2017, 25(7-8): 513-7.
DOI: 10.1177/0218492317731389. |
| [17] |
Ma S, Yan T, Liu D, et al. Minimally invasive esophagectomy in the lateral-prone position: Experience of 124 cases in a single center[J].
Thorac Cancer, 2018, 9(1): 37-43.
DOI: 10.1111/tca.2018.9.issue-1. |
| [18] |
Schroder W, Bruns CJ. Quality criteria for minimally invasive esophagectomy-a register analysis[J].
Chirurg, 2017, 88(11): 976.
DOI: 10.1007/s00104-017-0533-x. |
| [19] |
Fei XQ, Liao J, Wang DW, et al. Comparison of long-term outcomes of minimally invasive esophagectomy and open esophagectomy for esophageal squamous cell carcinoma[J].
Int J Clin Exp Med, 2016, 9(7): 14361-8.
|
| [20] |
Ninomiya I, Osugi H, Fujimura T, et al. Thoracoscopic esophagectomy with extended lymph node dissection in the left lateral position: technical feasibility and oncologic outcomes[J].
Dis Esophagus, 2014, 27(2): 159-67.
DOI: 10.1111/dote.2014.27.issue-2. |
| [21] |
Ahmadi N, Cinic A, Seely AJ, et al. Impact of surgical approach on perioperative and long-term outcomes following esophagectomy for esophageal cancer[J]. Surgical Endoscopy, 2017 Oct 24. doi: 10.1007/s00464-017-5881-6. [Epub ahead of print]
http://www.ncbi.nlm.nih.gov/pubmed/29067584
|
| [22] |
Matsutani N, Sawabata N, Yamaguchi M, et al. Does lung cancer surgery cause circulating tumor cells?-A multicenter, prospective study[J].
J Thorac Dis, 2017, 9(8): 2419-26.
DOI: 10.21037/jtd. |
| [23] |
Green TL, Cruse JM, Lewis RE, et al. Circulating tumor cells (CTCs) from metastatic breast cancer patients linked to decreased immune function and response to treatment[J].
Exp Mol Pathol, 2013, 95(2): 174-9.
DOI: 10.1016/j.yexmp.2013.06.013. |
| [24] |
Belani CP, Dahlberg SE, Rudin CM, et al. Vismodegib or cixutumumab in combination with standard chemotherapy for patients with extensive-stage small cell lung cancer: A trial of the ECOG-ACRIN Cancer Research Group(E1508)[J].
Cancer, 2016, 122(15): 2371.
DOI: 10.1002/cncr.30062. |
| [25] |
Zhao S, Liu Y, Zhang Q, et al. Erratum to:the prognostic role of circulating tumor cells(CTCs)detected by RT-PCR in breast cancer: a meta-analysis of published literature[J].
Breast Cancer Res Treat, 2011, 130(3): 809-16.
DOI: 10.1007/s10549-011-1379-4. |
| [26] |
Khoo BL, Lee SC, Kumar P, et al. Short-term expansion of breast circulating cancer cells predicts response to anti-cancer therapy[J].
Oncotarget, 2015, 6(17): 15578-93.
|
| [27] |
Pizon M, Schott D, Pachmann U, et al. The number of tumorspheres cultured from peripheral blood is a predictor for presence of metastasis in patients with breast cancer[J].
Oncotarget, 2016, 7(30): 48143-54.
|
| [28] |
Biere SS, Henegouwen M, Maas KW, et al. Minimally invasive versus open esophagectomy for patients with esophageal cancer:a multicenter, open-label, randomized controlled trial[J].
Lancet, 2012, 379(9829): 1887.
DOI: 10.1016/S0140-6736(12)60516-9. |
| [29] |
Taioli E, Schwartz RM, Lieberman-Cribbin W, et al. Quality of life after open or minimally invasive esophagectomy in patients with esophageal cancer-A systematic review[J].
Semin Thorac Cardiovasc Surg, 2017, 29(3): 377-90.
DOI: 10.1053/j.semtcvs.2017.08.013. |
| [30] |
Weksler B, Sullivan JL. Survival after esophagectomy: a propensitymatched study of different surgical approaches[J].
Ann Thorac Surg, 2017, 104(4): 1138-46.
DOI: 10.1016/j.athoracsur.2017.04.065. |
 2018, Vol. 38
2018, Vol. 38

