2. 南方医科大学普宁人民医院药剂科,广东 普宁 515300;
3. 南方医科大学南方医院肿瘤科,广东 广州 510515
2. Department of Pharmacy, Puning Peoples' Hospital, Southern Medical University, Puning 515300, China;
3. Department of Oncology, Nanfang Hospital, Southern Medical University, Guangzhou 510515, China
在肿瘤防治中,疫苗是研究的热点。当前肿瘤疫苗有多种形式,如全细胞疫苗、蛋白/肽疫苗、DNA疫苗等[1-2]。肿瘤细胞衰老时会发生一系列的变化,包括形态改变,衰老相关β-半乳糖苷酶(SA-β-gal)在pH值为6时仍具有较高活性[3-4],其虽处于生长停滞状态,但却能分泌独特的细胞因子,对机体免疫有正性刺激作用。这表明,衰老肿瘤细胞具有作为新型疫苗的潜力,其抗肿瘤效应令人期待。
在肿瘤微环境和宿主关系的研究中,发现肿瘤微环境会随着治疗的开展而改变,进而促使肿瘤细胞免疫逃逸,削弱治疗的效果[5-6]。微环境诱导肿瘤细胞抵抗机体免疫功能的机制有很多,而PD-1/PDL-1是主要通路之一[7-8]。在正常机体中,PD-1/PDL-1信号通路起着负性免疫调节作用,能维持免疫功能的平衡,避免免疫过强而导致自身免疫性疾病的发生。然而,对于肿瘤细胞而言,该通路是其自我保护,避免被免疫细胞清除的机制。学者们在对系统性红斑狼疮、慢性肝炎等患者的研究中也观察到了PD-1/PDL-1通路调节失衡,进而在免疫系统过度激活的患者血液中检测到sPD-1的存在[9-11]。进一步的研究揭示了sPD-1的性质,其本质上是PD-1的可溶性形式,能够被分泌至胞外,具有封闭细胞表面的PD-L1分子的活性,从而能阻断PD-1/PDL-1调节机制,所以导致机体免疫系统持续、过强激活[12-13]。
显然,疫苗的使用会改变机体的肿瘤微环境,有可能会引发肿瘤细胞PD-L1表达上调,进而阻碍肿瘤抗原呈递乃至T细胞激活的过程,导致疫苗失效[14-16]。这意味着衰老肿瘤细胞疫苗对肿瘤的防治效果也将面临着PD-1/PD-L1通路的限制。因此,我们设想将sPD-1与衰老肿瘤细胞疫苗联合起来,通过慢病毒感染4T1细胞,使衰老的4T1细胞自身分泌sPD-1,在小鼠模型上验证衰老肿瘤细胞作为疫苗的潜能的同时,验证sPD-1对衰老肿瘤细胞疫苗的增强作用,这在国内罕有报导,具有研究前景。
1 材料和方法 1.1 主要材料与试剂雌性Balb/c小鼠购自南方医科大学实验动物中心;4T1细胞购自武汉大学保藏中心;sPD-1过表达慢病毒购自上海吉凯基因公司;胎牛血清、1640高糖培养基购自美国Gibico;PARP抑制剂Veliparib购自美国Selleck;SA-β-gal试剂盒购自美国Cell Signaling Technology;Trizol、Prime Script RT reagent试剂盒、SYBR Primix Ex Taq TM试剂盒购自大连TaKaRa;引物购自上海Invitrogen;sPD-1抗体购自美国Sigma;Cell Counting Kit-8购自日本同仁公司;干扰素γ购自美国Sigma;PD-1、PD-L1流式细胞术抗体购自英国Abcam;日本Ix71倒置荧光显微镜;美国Varian2100直线加速器;SpectraMax M5多功能酶标仪。
1.2 方法 1.2.1 细胞培养配置含有10%胎牛血清的1640高糖培养基,用于培养小鼠乳腺癌细胞株4T1,5% CO2培养箱,37 ℃培养,2~3 d更换培养基,细胞密度达85%则传代。
1.2.2 干扰素γ刺激4T1细胞表达PD-L1将对数生长期的细胞接种于6孔板,每孔接种5×104细胞,用含有不同浓度干扰素(5、10、20、30 ng/mL)的培养液,5% CO2培养箱,37 ℃培养,24 h后收集细胞,PBS洗2遍,加入PD-L1流式抗体,4 ℃孵育30 min后检测PD-L1阳性细胞比例。
1.2.3 sPD-1过表达慢病毒感染将对数生长期的细胞接种于六孔板,每孔接种6×104细胞,加入sPD-1过表达慢病毒,5%CO2培养箱,37 ℃培养,8 h后更换培养基,72 h后倒置荧光显微镜下观察荧光表达情况。感染后细胞标志为4T1/sPD-1。
1.2.4 CCK8细胞增殖实验将对数生长期细胞4T1、4T1/sPD-1接种于96孔板,每孔接种2×103细胞,设置5个复孔,5% CO2培养箱,37 ℃培养。换液时,每孔换用100 μL含有10% CCK8试剂培养液,继续培养,于换液4 h后终止培养并用酶标仪检测吸光度值(A),记录第1、2、3、4、5天A值,操作过程注意避光。
1.2.5 荧光定量PCR将4T1、4T1/sPD-1细胞接种于六孔板,细胞密度达90%后,用Trizol提取各组细胞总RNA,计算浓度及纯度。20 μL体系中加入1 μg RNA,去除基因组DNA,逆转录获得cDNA:37 ℃ 15 min,85 ℃ 5 s,60 ℃ 10 min。根据说明书配置PCR反应液,用LightCycler 480 System Real Time PCR扩增仪进行两步法扩增:95 ℃ 30 s,95 ℃ 5 s,60 ℃ 20 s,40个循环,检测荧光强度。相对定量结果采用2-△△CT方法分析。
1.2.6 蛋白质免疫印记将4T1、4T1/sPD-1细胞接种于六孔板,细胞密度达90%后,收集培养基及细胞至1.5 mL的离心管中,每106细胞加入裂解液50 μL,冰上裂解30 min,4 ℃ 12 000 r/min离心15 min,收集上清,为所需裂解蛋白。用Bradford法测定蛋白含量,取50 μg蛋白用12%的聚丙烯酰胺凝胶进行电泳。用半干式转膜仪将蛋白转移到硝酸纤维膜上,用含5%脱脂奶粉封闭1 h,加入一抗4 ℃平摇过夜,用PBST缓冲液洗膜2次,10 min/次,加入二抗,室温孵育50 min,用PBST缓冲液洗膜4次,10 min/次。洗膜后加入ECL发光液,在暗室中压片、显影。
1.2.7 流式细胞术检测PD-1阳性细胞比例将4T1/ sPD-1用无血清培养基培养4 h,收集上清备用。干扰素γ刺激4T1细胞,24 h后收集细胞,PBS洗2遍,加入4T1 /sPD-1上清,室温孵育30 min,加入PD-1抗体,4 ℃孵育30 min,PBS洗2遍,上机检测。
1.2.8 诱导细胞衰老并β-半乳糖苷酶染色将4T1、4T1/ sPD-1细胞接种于六孔板,每孔接种5×104细胞,培养23 h后,用含有10 μmol/L Veliparib的培养液培养1 h,美国直线加速器进行照射,吸收剂量为9 Gy。2~3 d用含10 μmol/L Veliparib的培养液半换液。细胞照射后第4天进行SA-β-gal原位染色。
1.2.9 皮下移植瘤模型建立取处于对数生长期的小鼠乳腺癌细胞4T1,胰酶消化,PBS洗涤2次,调整细胞浓度为1×107/mL,于小鼠右后肢皮下注射100 μL细胞悬液。1.2.10疫苗效果验证预防性实验:先予小鼠左后肢接种衰老细胞疫苗(×106),3 d后小鼠右后肢建立皮下移植瘤模型;治疗性:在建立皮下移植瘤模型,3 d后给予小鼠左侧后肢皮下接种衰老细胞疫苗(×106);实验分组:PBS对照组、4T1 STCV组、4T1/sPD1 STCV组。记录小鼠肿瘤发生情况。
1.3 统计分析采用SPSS 20软件进行统计学分析,数据用均数±标准差表示,组间比较采用单因素方差分析,检验水准α=0.05,P < 0.05为差异有统计学意义。
2 结果 2.1 干扰素γ刺激后4T1细胞PD-L1表达上调4T1细胞有(0.72±0.17)%群体表达PD-L1分子。在含有干扰素γ的培养基培养下,4T1乳腺癌细胞PD-L1呈现高表达(图 1A)。干扰素γ能够诱导4T1细胞PD-L1表达上调,且与浓度成正性相关,在含有5、10、20、30 ng/mL干扰素γ条件培养下,PD-L1+ 4T1细胞比例分别为(17.405 ± 1.61)%,(26.8 ± 1.76)%,(61.90 ± 0.87)%和(84.80±1.03)%(P < 0.001,图 1B)。
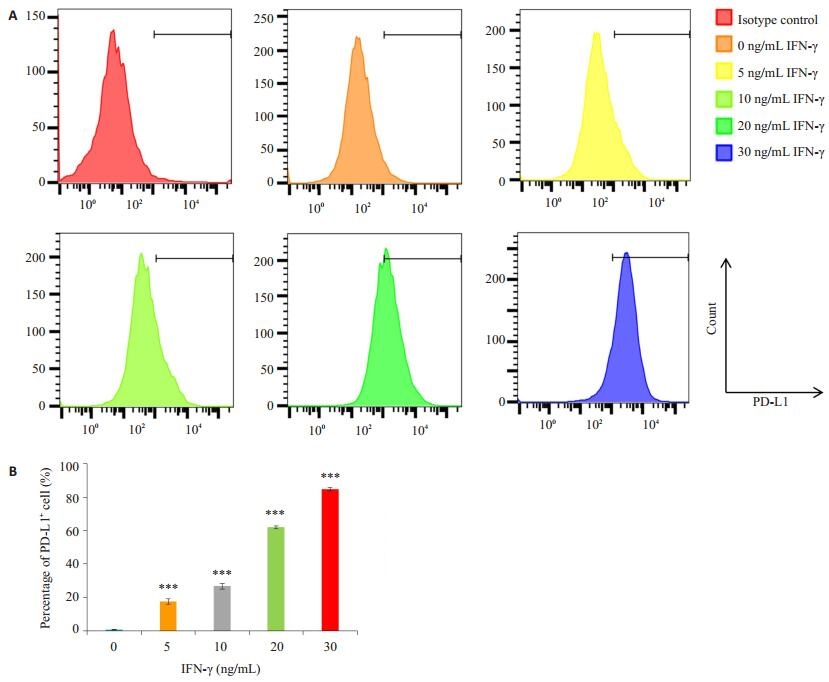
|
图 1 干扰素γ刺激4T1细胞24 h后PD-L1表达情况 Figure 1 Expression of PD-L1 in 4T1 cells detected by flow cytometry. A: 4T1 cells were treated with different concentrations of IFN-γ for 24 h; B: Percentage of PD-L1+ cells. ***P < 0.001 vs Control group. |
4T1细胞在sPD-1过表达慢病毒感染72 h后,荧光显微镜视野下可见强烈的绿色荧光(图 2A)。将感染的细胞收集后运用实时定量PCR,免疫蛋白印记检测sPD-1的表达,结果如图 2B-C,在4T1/sPD-1细胞,sPD-1在mRNA水平上表达显著上调(P < 0.001),并且在蛋白质水平的验证上也能见到明显的条带,而4T1细胞作为对照细胞,无论是在mRNA或是蛋白质水平均未能检测到sPD-1相应产物。
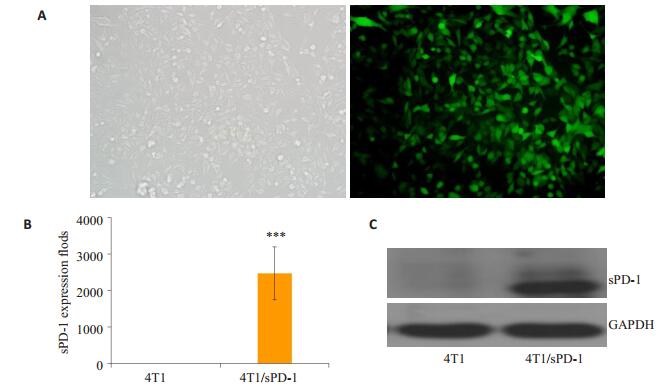
|
图 2 4T1/sPD-1稳转细胞株的建立 Figure 2 Establishment of 4T1/sPD-1 cell line. A: 4T1 cells tranfected by sPD-1encoding lentivirus observed under fluorescence microscope (Original magnification: ×200). B: Expressions of sPD-1 at mRNA level in 4T1 and 4T1/sPD-1 cells; C: Expressions of sPD-1 at protein level in 4T1 and 4T1/ sPD-1 cells. ***P < 0.001 vs Control group. |
为研究慢病毒感染是否对细胞增殖能力造成影响,研究采用CCK8实验比较4T1细胞与4T1/sPD-1细胞的增殖活力。图 3增殖曲线表明,在第1~5天的观察期内,4T1/sPD-1细胞在增殖能力方面与4T1细胞相仿(P>0.05)。
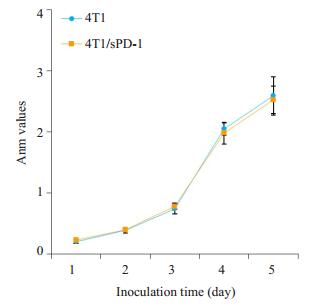
|
图 3 4T1、4T1/sPD-1细胞增殖能力比较 Figure 3 Comparison of proliferation activity in 4T1 and 4T1/sPD-1 cells. |
用干扰素γ诱导4T1细胞用PD-L1表达上调后,我们用流式细胞术检测其PD-1阳性细胞比例。结果如图 4,PD-1+ 4T1细胞比例低,约(6.893±0.271)%。在加入4T1/sPD-1培养上清后,PD-1阳性细胞比例明显升高,达(55.450±0.555)%(P < 0.001)。
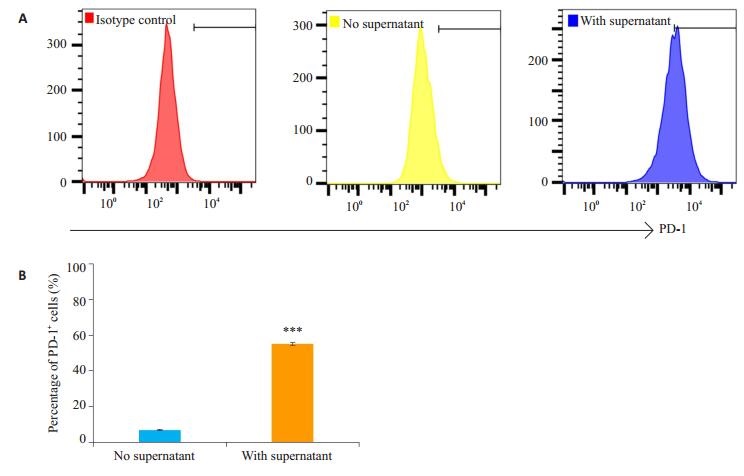
|
图 4 干扰素γ处理后各组4T1细胞PD-1阳性比例 Figure 4 Percentage of PD-1+ cells in IFN-γ pre-treated 4T1 cells. A: 4T1 cells pre-treated with IFN-γ were detected for PD-1 + ratio with or without 4T1/sPD-1 cells culture supernatant by flow cytometry. B: Percentage of PD-L+ cells. ***P < 0.001. |
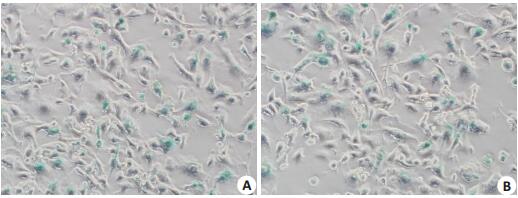
4T1细胞、4T1/sPD-1细胞在X线照射并在含有Veliparib的培养基培养下,出现衰老形态改变,体积明显变大、变扁平。在第4天,经过SA-β-gal染色后,4T1(图 5A)、4T1/sPD-1(图 5B)细胞均出现较多的蓝染的衰老细胞。
|
图 5 4T1、4T1/sPD-1细胞诱导衰老并染色 Figure 5 Senescence of 4T1 and 4T1/sPD-1 cells shown by β-galactosidase staining (×200). A: Senesnent 4T1 cells; B: Senesnent 4T1/sPD-1 cells. |
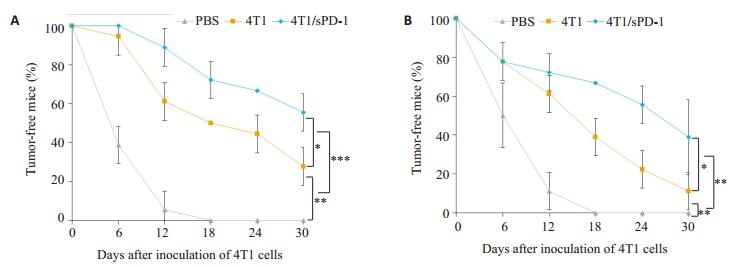
为验证疫苗的效果,Balb/c小鼠随机分组并予PBS、4T1 STCV、4T1/sPD-1 STCV处理。在预防肿瘤发生实验中,PBS组全部小鼠在早期均出现肿瘤生长,STCV组中,观察期中均有小鼠未发生肿瘤,其中4T1 STCV组有28.787%,4T1/sPD-1 STCV组中有55.556%(图 6A)。在治疗肿瘤实验中,PBS组小鼠短期内均出现肿瘤生长。而在观察期内,4T1/sPD-1 STCV组仍有38.89%无瘤小鼠,4T1 STCV组则只有11.111%的小鼠无肿瘤发生(图 6B)。
|
图 6 衰老肿瘤细胞疫苗预防及治疗性研究无瘤小鼠比例 Figure 6 Changes in the number of tumor-free mice receiving STCV for tumor prevention (A) and treatment (B). ***P < 0.001, **P < 0.01, *P < 0.05. |
乳腺癌尤其是三阴性乳腺癌患者,即使综合多学科手段进行治疗,临床上仍有不少发生肿瘤复发与转移[17-18]。本研究采用的研究对象小鼠乳腺癌4T1细胞,为三阴性乳腺癌细胞[19],侵袭性强,易转移,治疗手段相对较少,是探索新治疗方法的理想模型,结合当下衰老肿瘤细胞疫苗研究热点,可为乳腺癌防治提供新思路[20-21]。
在1961年,Hayflick便提出了细胞衰老的概念[22],随后的研究发现,肿瘤细胞在化疗药物、电离辐射等处理下会加速进入衰老状态[23]。衰老的肿瘤细胞处于特殊的存活状态,其增殖停滞, 而且还分泌多种生长因子和炎性细胞因子[24]。Meng等[14]通过衰老的B16恶性黑色素瘤细胞对小鼠黑色素瘤进行防治。但并不是所有的衰老细胞的分泌表型都能营造正性的免疫刺激环境,这取决于不同的癌种[25-26]。在前期研究中,我们发现单独使用电离辐射或是多聚二磷酸腺苷核糖聚合酶抑制剂Veliparib并不能有效诱导4T1细胞衰老,但是将二者联合起来处理细胞则能高效诱导4T1进入衰老状态。我们进一步用qPCR方法比较了衰老前后4T1的分泌表型,发现衰老后,IFN-β、TNF-α、CCL5、CXCL9、CXCL10等细胞因子的表达均上调,这提示4T1衰老细胞的分泌表型以免疫刺激正向作用为主[27]。结果表明,我们可以尝试利用这一免疫刺激效应来对小鼠乳腺癌进行防治。
本研究中我们对衰老4T1细胞疫苗的防治效果进行验证,观察到4T1衰老细胞的确能延缓小鼠乳腺癌的发生发展,在预防性疫苗研究中,4T1 STCV组中有28.787%的小鼠未发生肿瘤,而在治疗性效果的研究中,接受4T1 STCV处理的小鼠只有11.111%的无瘤小鼠。我们在早先的试验中证明了干扰素γ的存在会导致4T1细胞PD-L1的上调,而Verbrugge等[19]的研究,也证实了疫苗等治疗会促使机体肿瘤微环境的改变进而导致4T1细胞PD-L1表达上调而出现逃逸。因此,在肿瘤微环境中,衰老4T1细胞疫苗也会因为微环境内干扰素γ的影响而激活PD-1/PD-L1通路从而阻碍肿瘤抗原呈递与T细胞激活,导致疫苗疗效减弱。这也意味着STCV的效果还可以通过阻断该通路来进一步加强。
大量的研究将肿瘤疫苗与商业化的抗PD-1抗体联合起来治疗肿瘤。但是,商业化的抗体也有很大的缺点,其价格昂贵,副作用大,给患者带来巨大的负担。在自身免疫性疾病的患者中,同样存在着PD-1/PD-L1调节机制的失衡。在这我们进一步探索,尝试通过慢病毒将sPD-1基因导入4T1细胞内,并让4T1细胞稳定分泌sPD-1从而封闭自身上调的PD-L1分子,进而恢复衰老肿瘤细胞疫苗的免疫刺激效应[28-29]。sPD-1过表达慢病毒感染4T1细胞后,无论是在mRNA、蛋白质水平上均能检测sPD-1的产物,这意味着4T1/sPD-1细胞能稳定分泌sPD-1。同时,我们还用流式细胞术证实了4T1/ sPD-1所分泌的sPD-1的生物活性,4T1细胞中PD-1阳性比例升高提示了sPD-1能与PD-L1结合。此外,我们还用CCK8实验对4T1、4T1/sPD-1细胞增殖活性进行检测,排除慢病毒感染对细胞增殖能力的毒性影响。
在成功建立4T1/sPD-1细胞后,我们用X射线联合Veliparib对4T1、4T1/sPD-1细胞进行处理,经过SA-β-gal染色后,观察到大量的蓝染的变形细胞,提示4T1、4T1/sPD-1细胞大部分被诱导进入了衰老状态。我们进一步对设想进行验证,将衰老的细胞注射到小鼠皮下,对比4T1 STCV与4T1/sPD-1 STCV的疗效。从小鼠无瘤生存曲线上看,STCV表现出明显优于PBS的防治效果,其中,4T1 STCV虽然能延缓小鼠乳腺癌的发生发展,但是长期效果欠佳,随着时间的推移,最终在大部分小鼠上未能阻止肿瘤的进一步演变,其可能原因是受到PD-1/PD-L1通路的影响,4T1 STCV效果受到明显的抑制。而4T1/sPD-1 STCV因本身能表达sPD-1,在抗原呈递及T细胞的激活中能打破负性免疫机制的限制,故无论是在预防肿瘤的发生还是抑制肿瘤的生长则表现出更为高效的能力,在长期效果观察中,小鼠肿瘤生长抑制效果皆较4T1 STCV佳。
综上所述,STCV能在一定程度上抑制乳腺癌的发生发展,4T1/sPD-1 STCV因通过分泌sPD-1阻断PD-1/ PD-L1免疫逃逸机制,能更有效地呈递抗原并激活T细胞而成为更为高效的疫苗。本研究为临床上乳腺癌尤其是三阴性乳腺癌的防治策略提供了新的思路。
| [1] | Wagner SC, Ichim TE, Bogin V, et al. Induction and characterization of anti-tumor endothelium immunity elicited by ValloVax therapeutic cancer vaccine[J]. Oncotarget, 2017, 8(17): 28595-613. |
| [2] | Martin LS, Wolfer A, Harari A, et al. Cancer vaccines in ovarian cancer: how can we improve?[J]. Biomedicines, 2016, 4(2): e4020010: 1-28. |
| [3] | Munoz-Espin D, Serrano M. Cellular senescence: from physiology to pathology[J]. Nat Rev Mol Cell Biol, 2014, 15(7): 482-96. DOI: 10.1038/nrm3823. |
| [4] | Sikora E, Mosieniak G, Sliwinska MA. Morphological and functional characteristic of senescent cancer cells[J]. Curr Drug Targets, 2016, 17(4): 377-87. DOI: 10.2174/1389450116666151019094724. |
| [5] | Ogino S, Galon J, Fuchs CS, et al. Cancer immunology-analysis of host and tumor factors for personalized medicine[J]. Nat Rev Clin Oncol, 2011, 8(12): 711-9. DOI: 10.1038/nrclinonc.2011.122. |
| [6] | Zhou JY, Cheung AK, Tan ZW, et al. PD1-based DNA vaccine amplifies HIV-1 GAG-specific CD8(+) T cells in mice[J]. J Clin Invest, 2013, 123(6): 2629-42. DOI: 10.1172/JCI64704. |
| [7] | Zeng J, See AP, Phallen J, et al. Anti-PD-1 blockade and stereotactic radiation produce Long-Term survival in mice with intracranial gliomas[J]. Int J Radiat Oncol Biol Phys, 2013, 86(2): 343-9. DOI: 10.1016/j.ijrobp.2012.12.025. |
| [8] | Fu J, Malm IJ, Kadayakkara DK, et al. Preclinical evidence that PD1 blockade cooperates with cancer vaccine TEGVAX to elicit regression of established tumors[J]. Cancer Res, 2014, 74(15): 4042-52. DOI: 10.1158/0008-5472.CAN-13-2685. |
| [9] | Bommarito D, Hall C, Taams LS, et al. Inflammatory cytokines compromise programmed cell death-1(PD-1)-mediated T cell suppression in inflammatory arthritis through up-regulation of soluble PD-1[J]. Clin Exp Immunol, 2017, 188(3, SI): 455-66. DOI: 10.1111/cei.2017.188.issue-3. |
| [10] | Totsuka T, Kanai T, Uraushihara K, et al. Blockade of B7-H1 suppresses the development of chronic intestinal inflammation[J]. J Immunol, 2003, 171(8): 4156-63. DOI: 10.4049/jimmunol.171.8.4156. |
| [11] | Li N, Zhou ZH, Li F, et al. Circulating soluble programmed death-1 levels May differentiate immune-tolerant phase from other phases and hepatocellular carcinoma from other clinical diseases in chronic hepatitis B virus infection[J]. Oncotarget, 2017, 8(28): 46020-33. |
| [12] | Elhag OA, Hu XJ, Wen-Ying Z, et al. Reconstructed adenoassociated virus with the extracellular domain of murine PD-1 induces antitumor immunity[J]. Asian Pac J Cancer Prev, 2012, 13(8): 4031-6. DOI: 10.7314/APJCP.2012.13.8.4031. |
| [13] | He YF, Zhang GM, Wang XH, et al. Blocking programmed death-1 ligand-PD-1 interactions by local gene therapy results in enhancement of antitumor effect of secondary lymphoid tissue chemokine[J]. J Immunol, 2004, 173(8): 4919-28. DOI: 10.4049/jimmunol.173.8.4919. |
| [14] | Meng YR, Efimova EV, Hamzeh KW, et al. Radiation-inducible Immunotherapy for cancer: Senescent tumor cells as a cancer vaccine[J]. Mol Ther, 2012, 20(5): 1046-55. DOI: 10.1038/mt.2012.19. |
| [15] | Combe P, de Guillebon E, Thibault CA, et al. Trial watch: therapeutic vaccines in metastatic renal cell carcinoma[J]. Oncoimmunology, 2015, 4(5): e1001236:1-10. |
| [16] | Philips GK, Atkins M. Therapeutic uses of anti-PD-1 and anti-PDL1 antibodies[J]. Int Immunol, 2015, 27(1): 39-46. DOI: 10.1093/intimm/dxu095. |
| [17] | Au JL, Yeung BZ, Wientjes MG, et al. Delivery of cancer therapeutics to extracellular and intracellular targets: Determinants, barriers, challenges and opportunities[J]. Adv Drug Deliv Rev, 2016, 97(3): 280-301. |
| [18] | Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012[J]. Int J Cancer, 2015, 136(5): E359-86. DOI: 10.1002/ijc.29210. |
| [19] | Verbrugge I, Hagekyriakou J, Sharp LL, et al. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies[J]. Cancer Res, 2012, 72(13): 3163-74. DOI: 10.1158/0008-5472.CAN-12-0210. |
| [20] | Weiland T, Lampe J, Essmann F, et al. Enhanced killing of therapyinduced senescent tumor cells by oncolytic measles vaccine viruses[J]. Int J Cancer, 2014, 134(1): 235-43. DOI: 10.1002/ijc.v134.1. |
| [21] | Simova J, Sapega O, Imrichova T, et al. Tumor growth accelerated by chemotherapy-induced senescent cells is suppressed by treatment with IL-12 producing cellular vaccines[J]. Oncotarget, 2016, 7(34): 54952-64. |
| [22] | Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains[J]. Exp Cell Res, 1961, 25(4): 585-621. |
| [23] | Nardella C, Clohessy JG, Alimonti A, et al. Pro-senescence therapy for cancertreatment[J]. Nat Rev Cancer, 2011, 11(7): 503-11. DOI: 10.1038/nrc3057. |
| [24] | Loaiza N, Demaria M. Cellular senescence and tumor promotion: Is aging the key?[J]. Biochim Biophys Acta, 2016, 1865(2): 155-67. |
| [25] | Yoshimoto S, Loo TM, Atarashi K, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome[J]. Nature, 2013, 499(7456): 97-101. DOI: 10.1038/nature12347. |
| [26] | Rielland M, Cantor DJ, Graveline R, et al. Senescence-associated SIN3B promotes inflammation and pancreatic cancer progression[J]. J Clin Invest, 2014, 124(5): 2125-35. DOI: 10.1172/JCI72619. |
| [27] | 张佳和, 胡康, 杨子科, 等. X射线照射联合Veliparib诱导小鼠乳腺癌细胞衰老的相关研究[J]. 中华肿瘤防治杂志, 2016, 23(17): 1141-8. |
| [28] | Xiao H, Huang B, Yuan Y, et al. Soluble PD-1 facilitates 4-1BBLtriggered antitumor immunity against murine H22 hepatocarcinoma in vivo[J]. Clin Cancer Res, 2007, 13(6): 1823-30. DOI: 10.1158/1078-0432.CCR-06-2154. |
| [29] | Qiu H, Liu SP, Xie CH, et al. Regulating immunity and inhibiting tumor growth by the recombinant peptide sPD-1-CH50[J]. Anticancer Res, 2009, 29(12): 5089-94. |
 2018, Vol. 38
2018, Vol. 38

