2. 南方医科大学基础医学院人体组织胚胎学教研室,广东 广州 510515;
3. 南方医科大学珠江医院普通外科,广东 广州 510282
2. Department of Histology and Embryology, State Key Laboratory, Southern Medical University, Guangzhou 510515, China;
3. Department of General Surgery, Zhujiang Hospital, Southern Medical University, Guangzhou 510282, China
近年来,乳腺癌已成为全世界最常见的妇科恶性肿瘤之一,位居于我国部分大中城市女性恶性肿瘤发病率的第1位,尽管治疗手段的不断提高,仍有20%~30%的早期发病者进展为远处转移[1-2]。研究乳腺癌的浸润转移机制对于寻找临床治疗的分子靶点,提高患者生存率和延长生存时间具有重要的意义。乳腺癌细胞系全基因组转录谱的鉴定结果显示,上皮-间质转化(EMT)相关标志物的表达与其侵袭转移显著相关[3-6]。最新的研究表明,乳腺癌干细胞通过其自身的塑形能力在EMT过程中发挥重要作用,并影响肿瘤的转移和发展[7]。色素上皮衍生因子(PEDF)是一个能有效抑制新生血管形成的蛋白,与血管内皮生长因子(VEGF)等共同调控新生血管的发生过程。既往研究也显示PEDF蛋白的表达与微血管密度(MVD)和疾病预后明显相关[8]。前期的初步实验证实PEDF蛋白参与了乳腺浸润性导管癌组织EMT的调控[9],但对于PEDF蛋白如何调控EMT,降低肿瘤远处转移继而影响患者的预后的机制尚不清楚。因此本研究进一步加强PEDF对EMT特征及转移具体机制的研究,通过研究乳腺癌细胞与PEDF共培养之后肿瘤特性的变化,探索肿瘤微环境中PEDF蛋白在乳腺癌肿瘤转移中具有的潜在作用,为乳腺癌寻找靶向治疗基因提供帮助。
1 资料和方法 1.1 临床资料收集佛山市第一人民医院病理科2009年1月~ 2010年10月间经手术切除并且具有完整病史的女性浸润性乳腺癌石蜡包埋组织119例;所有患者术前均未进行放、化疗及内分泌治疗,另选取50例乳腺癌肿块5 cm以外的乳腺组织作为对照,组织学切片证实均为正常乳腺组织。
1.2 主要试剂鼠抗人PEDF单克隆抗体、兔抗人E-cadherin单克隆抗体均为Millipore产品;兔抗人vimentin单克隆抗体为Cell Signaling产品;SABC免疫组化试剂盒和DAB显色试剂盒均购于武汉博士德生物工程有限公司;人乳腺癌细胞系SK-BR-3由南方医科大学中心实验室研究所保存,Lenti-PacTM HIV Expression包装试剂盒购自Gene Copoeia,siRNA序列由Invitrogen合成;PEDF(产物长度240 bp)引物正义链:5'-GGA UUU CUA CUU GGA UGA ATT-3',反义链:5'-UUC AUC CAA GUA GAAAUC CTC-3'。GAPDH(产物长度227 bp)引物正义链:5'-ACC TGA CCT GCC GTC TAG AA-3',反义链:5'-TCC ACC ACC CTG TTG CTG TA-3'。所用引物由TaKaRa合成。
1.3 实验方法 1.3.1 PEDF、E-cadherin及vimentin免疫组化检测SABC法检测PEDF、E-cadherin及vimentin在乳腺浸润性导管癌中和正常组织中的表达。PBS代替一抗做阴性对照。按SABC试剂盒说明书操作。PEDF阳性结果判断:每张切片在400倍镜下随机选择10个视野,计数200个细胞,阳性细胞 < 10%为阴性,≥10%为阳性。E-cadherin阳性结果判断参考Tanaka改良定量记分法[10]。Vimentin阳性结果判断标准:≤20%判定为vimentin低表达;>20%判定为vimentin高表达。
1.3.2 细胞培养SK-BR-3细胞分为两组:空白对照组:普通培养基,不进行任何处理;实验组:PEDF条件培养基。细胞置37 ℃、5% CO2孵育箱中培养72 h后,进行下述实验。
1.3.3 SiRNA转染及慢病毒包装按照1:1比例将LipofectamineRNAiMAX和SiRNA稀释液混合(SiRNA逐滴加入LipofectamineRNAiMAX),4~6 h换1次培养基,将转染SiRNA-Reagent的细胞继续培养(37 ℃,5% CO2孵箱)48 h后,用荧光显微镜观察绿色荧光蛋白的表达,检测转染效率;293T慢病毒包装细胞系,依脂质体转染试剂说明进行细胞转染,2 d后荧光显微镜观察,7~10 d后收集细胞,37~80 ℃反复冻融4次,8000 r/min,10 min,4 ℃,取部分病毒上清再次感染SKBR-3细胞以扩增慢病毒重组子。
1.3.4 划痕实验取传代48 h长势良好的SK-BR-3细胞制备单细胞悬液,按2×106细胞每孔将细胞加入6孔板,于5% CO2培养箱内37 ℃孵育。用200 µL的枪头比着背面事先画好的横线划痕,D-Hank's缓冲液冲洗2次,除去划下的细胞,加入无血清培养基。镜下观察,拍照记录0时划痕处的变化。放入37 ℃ 5% CO2培养箱中继续培养。按6、12、36、48、60、72 h取样,拍照,观察细胞在划痕处的迁移情况。
1.3.5 Transwell小室法细胞侵袭实验参照Ries等[6]的方法,在Transwell小室中铺Matrigel制成人工基质胶层,取对照组和实验组各200 μL(2×105/mL)细胞悬液加入上室,下室为10%胎牛血清+RPMI 1640培养液,500 μL/孔,于37 ℃、5% CO2孵育箱中培养24 h,固定染色。每组实验做3个复孔,相同实验重复3次。高倍镜(200×)下随机对5个视野的细胞进行连续计数,取均值。1.3.6 Western blot检测SK-BR-3细胞中E-cadherin及vimentin的表达将实验组和空白组细胞培养72 h后,裂解并提取蛋白,Western blot检测E-cadherin和vimentin的表达。
1.4 统计学分析釆用SPSS18.0统计软件。描述性统计量以均数±标准差表示。多组间细胞迁移宽度采用方差分析法,划痕创面面积不同时间及组间整体比较采用析因设计的方差分析,多组间参数各单独效应分析采用单因素方差分析法。P < 0.05为差异有统计学意义。
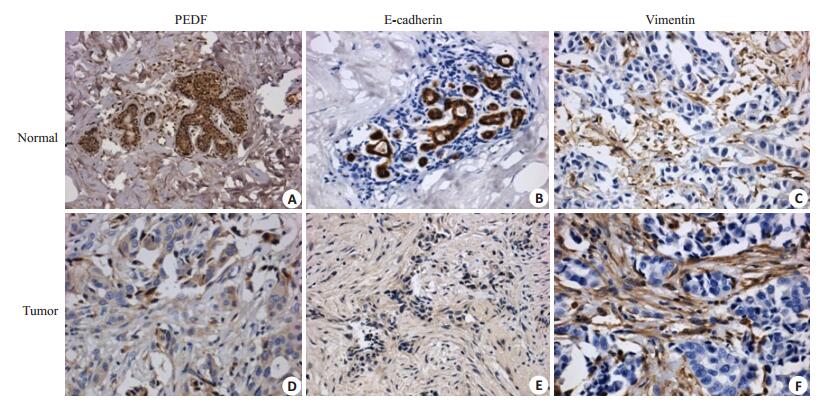
2 结果 2.1 PEDF、vimentin、E-cadherin在浸润性导管癌中的表达及与临床病理特征的关系(图 1)
|
图 1 PEDF、E-cadherin、vimentin在浸润性导管癌及癌旁常组织中的表达 Figure 1 Expression of PEDF, E-cadherin and vimentin in infiltrating ductal carcinoma and normal tissues (Original magnification: ×400). A: High expression of PEDF; B: Low expression of PEDF; C: High expression of E-cadherin; D: Low expression of E-cadherin; E: High expression of vimentin; F: Low expression of vimentin. |
PEDF蛋白在浸润性乳腺癌中的表达与肿瘤肿瘤大小有关(P < 0.05);而与患者年龄、TNM分期、组织学分级、ER受体、PR受体、腋窝淋巴结的转移无关(P>0.05)。Vimentin蛋白在浸润性乳腺癌中的表达与肿瘤TNM分期、肿瘤大小、腋窝淋巴结的转移、组织学分级、PR受体有关(P < 0.05);而与患者年龄、ER受体无关(P>0.05)。E-cadherin蛋白在浸润性乳腺癌中的表达与TNM分期、肿瘤大小、腋窝淋巴结的转移、PR受体有关(P < 0.05);而与患者年龄、组织学分级、ER受体无关(P>0.05,表 1)。
| 表 1 PEDF、vimentin、E-cadherin在乳腺癌中的表达及与临床病理特征的关系 Table 1 Expressions of PEDF, E-cadherin and vimentin in breast cancer and their relationship with the clinicopathological characteristics |
119例浸润性导管癌中,应用Spearman相关性分析发现:PEDF蛋白的表达与vimentin的表达呈明显负相关(r=-0.412,P < 0.001),而与E-cadherin的表达呈明显正相关(r=0.473,P < 0.001),vimentin与E-cadherin的表达呈明显负相关(r=-0.628,P < 0.001,表 2)。
| 表 2 PEDF、vimentin、E-cadherin蛋白在人乳腺浸润性导管癌组织中表达的相关性 Table 2 Relationship among the expressions of PEDF, E-cadherin and vimentin in breast cancer |
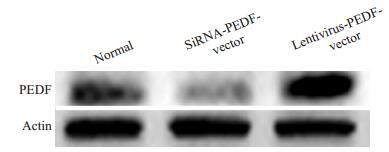
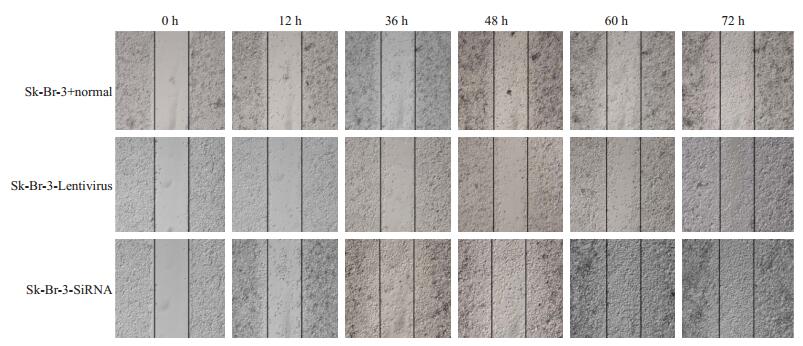
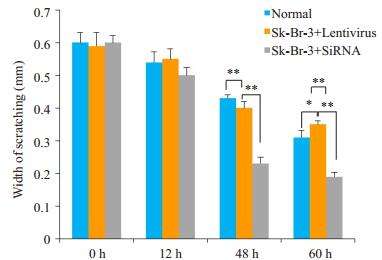
在细胞系和乳腺癌标本中验证了PEDF的表达,结果提示PEDF与乳腺癌的转移与EMT表型有着密切的关系。为了从功能学上验证这个假设,我们分别在SKBR-3细胞中下调PEDF(转染PEDF-siRNA)的表达和在SK-BR-3中扩增PEDF(转染Lentivirus-PEDF-vector)的表达,然后采用transwell实验检测了PEDF对这两株细胞系侵袭与迁移能力的影响,结果显示PEDF可以明显抑制乳腺癌细胞的侵袭和迁移能力。Western blot结果显示,SiRNA和Lentivirus-vector都能有效改变乳腺癌细胞系中PEDF的表达水平(图 2)。Transwell实验结果显示,与阴性对照相比,PEDF-siRNA可以明显增加SK-BR-3细胞的迁移与侵袭潜能,而LentivirusPEDF-vector则能明显抑制SK-BR-3细胞的侵袭与迁移能力(图 3)。这表明,PEDF的下调的确可以导致乳腺癌侵袭潜能的增强(图 4)。
|
图 2 SK-BR-3细胞中PEDF蛋白表达的Western blot分析 Figure 2 Analysis of PEDF protein expression by Western blotting. |
|
图 3 SK-BR-3细胞中PEDF蛋白划痕面积分析图 Figure 3 Scratch assay of SK-BR-3 cells with PEDF overexpression and knockdown. |
|
图 4 体外侵袭和迁移实验检测在SK-BR-3细胞中抑制和过表达PEDF蛋白后对细胞侵袭和迁移能力的影响 Figure 4 Effect of PEDF overexpression and knockdown on invasion and migration ability of SK-BR-3 cells. *P < 0.05, **P < 0.05. |
SK-BR-3细胞中PEDF的过表达,引起上皮细胞标志分子E-cadherin的表达显著增加,及间质细胞标志分子vimentin的表达显著下调;而与正常对照组和阴性对照组对比,PEDF-siRNA组的E-cadherin表达显著降低(P < 0.05),vimentin表达显著升高(P < 0.05)。而正常对照组和阴性对照组中E-cadherin、vimentin的表达无显著差异(P>0.05)。这说明,呈现间质样细胞表型的SKBR-3细胞在PEDF的作用下,发生了显著的EMT表型的逆转,向上皮样细胞表型转化(图 5)。

|
图 5 SK-BR-3细胞中过表达PEDF蛋白后EMT特征分子Western blot分析 Figure 5 Western blotting of EMT characteristics in SK-BR-3 cells with overexpression and knockdown. |
PEDF是一个发现于细胞外基质、相对分子质量为50 000的蛋白,它属于丝氨酸蛋白酶抑制剂家族,并且含有肝素和胶原结合位点[11-12]。它不但是一种强效的神经营养和血管生成抑制因子[13-14],而且PEDF表达和抑制肿瘤生长、减少转移、良好的预后相关[15-17]。PEDF蛋白通过层粘连蛋白受体AKT/ERK通路调低纤维连接蛋白,具有抑制乳腺癌的生长和转移双重特性[18]。前期研究已证明PEDF通过Fas/FasL死亡通路引起内皮细胞凋亡,而且减少了重要的VEGF的表达[19-20]。研究人员从乳腺癌细胞系MDA-MB-231培养基中得到的VEGF可诱导体外血管形成明显增加,之后添加PEDF可引起此血管形成相应减少。由此可知PEDF为VEGF促血管生成的抑制因子,进一步研究表明PEDF通过受体VEGFR-1通路来抑制VEGF血管生成作用[21]。并进一步研究表明VEGFR-1通过EMT作用促进了乳腺癌细胞系的侵袭和转移[22]。因此有必要对PEDF蛋白调控EMT及其在乳腺癌浸润转移中的作用及其调控机制因素进一步研究。
本研究结果显示,相对于高潜能转移的乳腺癌SKBR-3细胞系,PEDF蛋白在乳腺癌高转移潜能细胞系SK-BR-3中明显下调,本实验中采用Western blot验证了PEDF在乳腺癌细胞系SK-BR-3中的表达,结果与前期结果相一致。从分子水平分析,PEDF基因沉默后,乳腺癌SK-BR-3细胞的增殖能力、体外侵袭增强。细胞-基质粘附实验结果表明,PEDF表达的减少引起乳腺癌细胞SK-BR-3与基质粘附性的降低。
EMT的重要特性之一就是降低上皮细胞特性及提高间质细胞特性[23-24],上皮细胞逐渐失去细胞极性、细胞间黏附力减弱及细胞骨架重塑,EMT的发生不仅使细胞表型发生改变, 而且细胞标记物也发生改变:如Ecadherin、细胞角蛋白等的下调,间质样细胞标记物表达的上调如vimentin、N-cadherin等[25-28]。本研究发现,vimentin在乳腺癌中的高表达与远处转移、病理分期显著相关(P < 0.05)。研究发现随着乳腺癌病理分期的增加,E-cadherin蛋白的表达逐渐减弱,而vimentin则与其相反,且两者在乳腺癌组织中的表达呈负相关(r=-0.628,P < 0.001),从而证实了乳腺癌中存在EMT现象,此现象可能与乳腺癌侵袭和转移有关。另外,通过Spearman相关性分析发现,PEDF蛋白在乳腺癌组织中的表达与E-cadherin(r=0.473,P < 0.001)和vimentin(r=-0.412,P < 0.001)的表达均有显著相关性。PEDF有可能通过调控这些细胞标记物的表达,在乳腺癌的侵袭、转移过程中发挥作用。Western blot检测进一步验证发现,SK-BR-3细胞转染PEDF-siRNA质粒后E-cadherin表达显著降低,vimentin表达明显升高。相反,LentivirusPEDF-vector转染SK-BR-3细胞,免疫荧光染色显示SK-BR-3细胞骨架形态由间质样变为上皮样,Ecadherin表达上调,vimentin表达下调。
目前国内外对EMT相关的促进因子、转录因子均有所研究,尤其是在乳腺癌方面,金属蛋白酶、miRNA等在调控EMT方面取得了一定的研究进展[29-30]寻找一个靶向基因研究其在乳腺癌调控EMT的方面研究甚少。此次研究首次探讨PEDF蛋白在乳腺癌中表达与乳腺癌上皮间质转化的关系及其在乳腺癌浸润转移中的作用,本研究的意义在于说明PEDF能够参与并作用乳腺癌细胞上皮向间质转化,抑制乳腺癌细胞迁移侵袭。为临床上预防肿瘤细胞的侵袭和转移提供依据和可能的干预靶点。
| [1] | Siegel RL, Miller KD. Cancer statistics, 2016[J]. CA Cancer J Clin, 2016, 66(5): 7-30. |
| [2] | Papageorgis P, Ozturk S, Lambert AW, et al. Targeting IL13ralpha2 activates STAT6-TP63 pathway to suppress breast cancer lung metastasis[J]. Breast Cancer Res, 2015, 17(17): 98. |
| [3] | Smith BN, Bhowmick N. Role of EMT in metastasis and therapy resistance[J]. J Clin Med, 2016, 5(2): E17. DOI: 10.3390/jcm5020017. |
| [4] | Matysiak M, Kapka-Skrzypczak L, Jodlowska-Jedrych B, et al. EMT promoting transcription factors as prognostic markers in human breast cancer[J]. Arch Gynecol Obstet, 2017, 295(4): 817-25. DOI: 10.1007/s00404-017-4304-1. |
| [5] | Hollier BG, Evans K, Mani SA. The Epithelial-to-Mesenchymal transition and Cancer stem cells: a coalition against cancer therapies[J]. J Mammary Gland Biol Neoplasia, 2009, 14(1): 29-43. |
| [6] | Blick T, Widodo E, Hugo H, et al. Epithelial mesenchymal transition traits in human breast cancer cell lines[J]. Clin Exp Metastasis, 2008, 25(6): 629-42. DOI: 10.1007/s10585-008-9170-6. |
| [7] | Wang SS, Jiang J, Liang XH, et al. Links between cancer stem cells and epithelial-mesenchymal transition[J]. Onco Targets Ther, 2015, 8(8): 2973-80. |
| [8] | Dan Z, Cheng SQ, Ji HF, et al. Evaluation of protein pigment epithelium derived factor (PEDF) and microvessel density(MVD)as prognostic indicators in carcinoma breast[J]. J Cancer Res Clin Oncoly, 2010, 136(11): 1719-27. DOI: 10.1007/s00432-010-0830-y. |
| [9] | Dan Z, Min Z, Xu PC, et al. Expression of pigment EpitheliumDerived factor increases favorable prognosis and correlates Epithelial-Mesenchymal transition related factor in infiltrating ductal breast carcinoma[J]. Oncol Lett, 2016, 11(1): 116-24. DOI: 10.3892/ol.2015.3880. |
| [10] | Tanaka K, Iwamoto S, Gon G, et al. Expression of survivin and its relationship to loss of apoptosis in breast carcinomas[J]. Clin Cancer Res, 2000, 6(1): 127-34. |
| [11] | Protiva P, Gong J, Sreekumar B, et al. Pigment epithelium-derived factor (PEDF) inhibits Wnt/β-catenin signaling in the liver[J]. Cell Mol Gastroenterol Hepatol, 2015, 1(5): 535-49. DOI: 10.1016/j.jcmgh.2015.06.006. |
| [12] | Ladhani O, Sanchez-Martinez C, Orgaz JL, et al. Pigment epitheliumderived factor blocks tumor extravasation by suppressing amoeboid morphology and mesenchymal proteolysis[J]. Neoplasia, 2011, 13(7): U86-633. |
| [13] | Filleur S, Nelius T, de Riese W, et al. Characterization of PEDF: A Multi-Functional serpin family protein[J]. J Cell Biochem, 2009, 106(5): 769-75. DOI: 10.1002/jcb.v106:5. |
| [14] | He XE, Cheng R, Benyajati S, et al. PEDF and its roles in physiological and pathological conditions: implication in diabetic and hypoxia-induced angiogenic diseases[J]. Clin Sci (Lond), 2015, 128(11): 805-23. DOI: 10.1042/CS20130463. |
| [15] | Belkacemi L, Zhang SX. Anti-tumor effects of pigment epitheliumderived factor (PEDF): implication for Cancer therapy. A minireview[J]. J Exp Clin Cancer Res, 2016, 35(3): 4. |
| [16] | Han J, Guo J. Current evidence and potential mechanisms of therapeutic action of PEDF in cervical cancer treatment[J]. Curr Mol Med, 2015, 15(5): 446-55. DOI: 10.2174/1566524015666150630124447. |
| [17] | Alcantara MB, Dass CR. Pigment epithelium-derived factor as a natural matrix metalloproteinase inhibitor: a comparison with classical matrix metalloproteinase inhibitors used for cancer treatment[J]. J Pharm Pharmacol, 2014, 66(7): 895-902. DOI: 10.1111/jphp.2014.66.issue-7. |
| [18] | Hong HH, Zhou T, Fang SH, et al. Pigment epithelium-derived factor (PEDF) inhibits breast cancer metastasis by down-regulating fibronectin[J]. Breast Cancer Res Treat, 2014, 148(1): 61-72. DOI: 10.1007/s10549-014-3154-9. |
| [19] | Kawaguchi T, Yamagishi SI, Sata M. Structure-function relationships of PEDF[J]. Curr Mol Med, 2010, 10(3): 302-11. DOI: 10.2174/156652410791065255. |
| [20] | Zhang H, Wei TT, Jiang X, et al. PEDF and 34-mer inhibit angiogenesis in the heart by inducing tip cells apoptosis via upregulating PPAR-gamma to increase surface FasL[J]. Apoptosis, 2016, 21(1): 60-8. DOI: 10.1007/s10495-015-1186-1. |
| [21] | Falero-Perez J, Park SY, Sorenson CM. PEDF expression affects retinal endothelial cell proangiogenic properties through alterations in cell adhesive mechanisms[J]. Am J Physiol Cell Physiol, 2017, 313(4): C405-20. DOI: 10.1152/ajpcell.00004.2017. |
| [22] | Qian N, Liu CG, Lei H, et al. Vascular endothelial growth factor receptor-1 activation promotes migration and invasion of breast cancer cells through epithelial-mesenchymal transition[J]. PLoS One, 2013, 8(6): e65127. DOI: 10.1371/journal.pone.0065127. |
| [23] | Kovacic JC, Mercader N, Torres M, et al. Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition: from cardiovascular development to disease[J]. Circulation, 2012, 125(14): 1795-808. DOI: 10.1161/CIRCULATIONAHA.111.040352. |
| [24] | Maier J, Traenkle B, Rothbauer U. Visualizing Epithelial-Mesenchymal transition using the chromobody technology[J]. Cancer Res, 2016, 76(19): 5592-6. DOI: 10.1158/0008-5472.CAN-15-3419. |
| [25] | Kaufhold S, Bonavida B. Central role of Snail1 in the regulation of EMT and resistance in cancer: a target for therapeutic intervention[J]. J Exp Clin Cancer Res, 2014, 33(2): 62. |
| [26] | Lee GA, Hwang KA, Choi KC. Roles of dietary phytoestrogens on the regulation of Epithelial-Mesenchymal transition in diverse cancer metastasis[J]. Toxins (Basel), 2016, 8(6): E162. DOI: 10.3390/toxins8060162. |
| [27] | Zhou XM, Zhang H, Han X. Role of epithelial to mesenchymal transition proteins in gynecological cancers: pathological and therapeutic perspectives[J]. Tumour Biol, 2014, 35(10): 9523-30. DOI: 10.1007/s13277-014-2537-1. |
| [28] | Kim YS, Yi BR, Kim NH, et al. Role of the epithelial-mesenchymal transition and its effects on embryonic stem cells[J]. Exp Mol Med, 2014, 46(3): e108. |
| [29] | Rafael D, Doktorovova S, Florindo HF, et al. EMT blockage strategies: targeting Akt dependent mechanisms for breast cancer metastatic behaviour modulation[J]. Curr Gene Ther, 2015, 15(3): 300-12. DOI: 10.2174/1566523215666150126123642. |
| [30] | Ding XM. MicroRNAs: regulators of cancer metastasis and epithelialmesenchymal transition (EMT)[J]. Chin J Cancer, 2014, 33(3): 140-7. DOI: 10.5732/cjc.013.10094. |
 2018, Vol. 38
2018, Vol. 38
