2. 河南中医药大学中医外科,河南 郑州 450000
2. Surgery of Traditional Chinese Medicine, Henan University of Chinese Medicine, Zhengzhou 450046, China
结直肠癌是最常见的消化道癌症之一,除了遗传因素,饮食因素和一些结直肠癌相关的癌前病变如直肠腺瘤、非腺瘤性结直肠息肉病和炎症性肠病都可引发结直肠癌[1-4]。其病发率和病死率呈上升趋势,并且女性发病率逐年增加,结直肠癌的诊疗仍然任重道远[5-6]。脾酪氨酸激酶(SYK)是一种非受体型蛋白酪氨酸激酶,具有抑癌作用,在乳腺癌中的研究比较多,SYK的表达减少或消失,与乳腺癌的侵袭性增强相关[7-9]。骨髓瘤中,SYK同样抑制细胞增殖和迁移[10]。另外,胃癌的恶化和淋巴结转移也与SYK表达量的减少有关[11]。然而,对于SYK表达量与结直肠癌细胞的增殖和凋亡之间的关系则仍没有相关文献报道。因此,本实验旨在通过过表达SYK探讨其对结直肠癌细胞增殖和凋亡的影响,为结直肠癌的防治提供一定的参考价值。
1 材料和方法 1.1 主要试剂实验材料:HCT116、Sw480和FHC细胞购自美国ATCC;胎牛血清、DMEM购自美国Gibco;SYBR Premix Ex Taq Ⅱ、TRIZOL和质粒小提试剂盒购自大连宝生物公司;XhoⅠ、Turbofect、EcoRI和反转录试剂盒购自赛默飞世尔科技公司;SYK鼠单抗和Fra-1鼠单抗购自英国Abcam;GAPDH鼠单抗和辣根过氧化物酶标记的羊抗鼠二抗购自北京博奥森公司;DH5a细胞购自天根生化科技(北京)公司;MTT购自上海生工公司;BCA试剂盒购自美国Pierce。
1.2 细胞的培养及组织采集结直肠癌细胞HCT116和Sw480以及正常的结肠细胞FHC均由含有10%胎牛血清(FBS)的DMEM培养基,于5% CO2、37 ℃的细胞培养箱中培养,待细胞融合度达80%即可传代。
20例新诊断的结直肠癌病例的直结肠癌组织(10例)和癌旁组织(10例)由河南省中医院提供,并通过了伦理审查委员会的批准。患者平均年龄65岁(50~78岁)。另外,癌旁组织采集自离癌变组织至少有1 cm距离处,采集的组织立即冷冻或存放于液氮中。
1.3 qRT-PCR检测组织在液氮中磨碎,匀浆裂解后,Trizol法提取总RNA,利用反转录试剂盒将总RNA反转成cDNA,进行qRT-PCR反应。GAPDH为内参基因。反应程序:预变性94 ℃ 2 min;94 ℃ 30 s,60 ℃ 1 min,共40个循环;后延伸72 ℃ 10 min。反应体系20 μL含有10 μL SYBR Premix Ex Taq Ⅱ,1 μL cDNA。PCR相关引物为SYK:Sense primer:5'-TGTCAAGGATAAGAACATCATAG-3',Anti-sense primer:5'-CACCACGTCATAGTAGTA ATTG-3';GAPDH:Sense primer:5'-CGTCTTCACCA CCATGGAGA-3',Anti-sense primer:5'-CGGCCATCA CGCCACAGTTT-3'Fra-1:Sense primer:5'-GAGTAA GGCGCGAGCGGAACAA-3',Anti-sense primer:5'-TGGAACATAGAGGGAAAGGGGTCC-3'。2-ΔΔCT法计算基因相对表达量,每个样品设置3个重复。
1.4 蛋白免疫印记(Western blot)收集需要检测的细胞并裂解,提取其中的蛋白质并且利用BCA试剂盒检测其浓度。取25 μg蛋白样品进行SDS-PAGE(10%),蛋白电泳分离后利用半干法将蛋白电转移至硝酸纤维素膜。封闭液(5%脱脂奶粉溶液)封闭硝酸纤维素膜2.5 h,然后用相应一抗SYK鼠单抗(1:500)、Fra-1鼠单抗(1:500)、GAPDH鼠单抗(1:1000)4 ℃过夜孵育。PBS洗去未结合的抗体,辣根过氧化物酶标记的羊抗兔二抗(1:1000)于室温下孵育1 h,利用凝胶成像系统观察结果。GAPDH为内参对照蛋白,实验重复3次取平均值。
1.5 构建重组质粒pcDNA.3.1-SYK及细胞转染Trizol法提取9×105个FHC细胞的总RNA,按照反转录试剂盒的说明书,将其反转录成cDNA,以cDNA为模板,利用PCR方法扩增SYK全长Sense primer:5'-CGTACCTCGAGGCGGAATTCGCCACCATGGCCA G(下划线为XhoⅠ酶切位点);Anti-sense primer:5'-GGAATTCGGCCTCGAGTTAGTTCACCACGTCAT AGTAG(下划线为EcoRI酶切位点)。反应条件:94 ℃ 6 min;94 ℃ 30 s,56 ℃ 50 s,72 ℃ 1 min,35个循环;72 ℃ 10 min。将25 μL PCR反应产物进行琼脂糖凝胶电泳,XhoⅠ和EcoRI双酶切回收的DNA和pcDNA.3.1质粒并且连接,转化至感受态细胞DH5α中,挑选阳性克隆增殖重组质粒并将其提取,双酶切鉴定正确的质粒送上海英骏生物公司测序。利用Turbofect(7 μL)将测序正确的重组质粒(6 μg)和质粒pcDNA.3.1(6 μg)转染进入于6孔板中培养的融合度达70%的HCT116,Sw480细胞,操作步骤按照Turbofect操作说明书进行。转染48 h后利用qRT-PCR和Western Blot方法检测转染效率。分组情况如下。(1)pcDNA.3.1-SYK(HCT116):转染pcDNA.3.1-SYK到HCT116,过表达SYK;(2)pcDNA.3.1(HCT116):转染pcDNA.3.1空载体到HCT116中,作为对照组;(3)Normal(HCT116):正常HCT116细胞。(1)pcDNA.3.1-SYK(Sw480):转染pcDNA.3.1-SYK到Sw480,过表达SYK;(2)pcDNA.3.1(Sw480):转染pcDNA.3.1空载体到Sw480中,作为对照组;(3)Normal(Sw480):正常Sw480细胞。
1.6 Annexin-V FITC/PI检测细胞凋亡转染后细胞的凋亡情况参照(Annexin-V FITC/PI)试剂盒说明书进行检测。用12 μL Annexin-V FITC(1 µL/mL)于4 ℃孵育已用PBS预冷的细胞35 min,而后用PBS洗细胞,8 μL PI孵育8 min,利用流式细胞仪检测细胞的凋亡情况。
1.7 Caspase-3活性检测收获细胞,PBS洗涤,按照试剂盒(BD ApoAlert Caspase-3 Fluorescent Assay Kit)说明书对Caspase-3进行活性检测,记录结果。
1.8 细胞生长活力测定与BrdU检测按每孔5×104细胞接种于96孔板,5% CO2,37 ℃培养24 h后,将0.2 μg重组质粒和0.2 μg质粒pcDNA.3.1分别与0.4 μL Turbofect混匀,然后分别加入到200 μL不含血清的DMEM培养基,充分混合加入96孔板不同的细胞培养孔中,继续培养48 h后每孔加入20 μL MTT(5 mg/mL)培养4 h,每孔加入150 μL二甲基亚砜换掉之前的上清,溶解蓝紫色结晶甲瓒,用酶标仪测定A450 nm处吸光值。另外,Brdu试剂盒检测细胞增殖:96孔板接种细胞并转染后,弃上清,每孔加入200 μL修复变性溶液室温反应30 min,弃上清,加95 μL antiBrdu-POD工作液后室温反应90 min,加入100 μL底物,室温反应15 min后用2 mol/L硫酸终止反应,于450 nm波长检测A值。实验重复3次,取平均数作为实验结果。
1.9 统计学分析采用SPSS 16.0软件进行统计学分析,数值均采用均数±标准差。多组之间采用单因素方差分析,多组之间两两比较采用SNK-Q检验的方法,以P<0.05为差异有统计学意义。
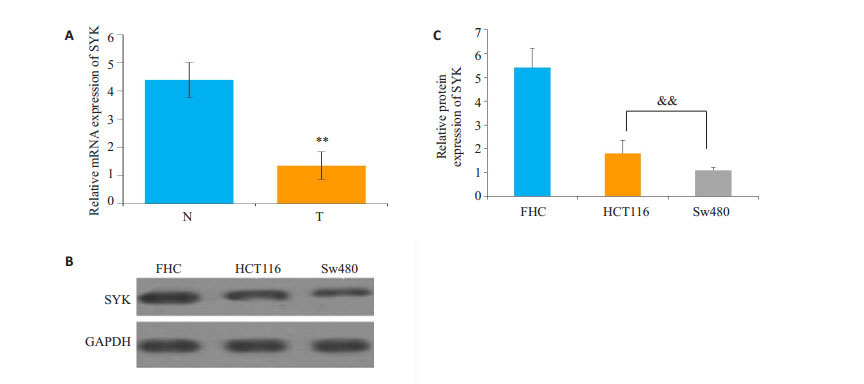
2 结果 2.1 结直肠癌组织和细胞中SYK表达量降低本实验应用qRT-PCR和Western blot分别检测结直肠癌组织、癌旁组织中SYK的mRNA和结直肠癌细胞HCT116、Sw480,正常结肠细胞FHC中SYK的蛋白表达量。结果显示,结直肠癌组织中SYK mRNA表达量较癌旁组织显著下调。另外在HCT116和Sw480细胞中,SYK蛋白表达量较FHC细胞显著下调(P<0.01,图 1)。
|
图 1 结直肠癌SYK表达量的变化 Figure 1 Expression of SYK in colorectal cancer tissue (T) and adjacent tissues (N). A: mRNA expression of SYK; B: Western blotting of SYK protein in the tissues; C: Quantitative analysis of Western blotting results. FHC: Normal colon cells; **P < 0.01 vs adjacent tissue; && P < 0.01 vs FHC. |
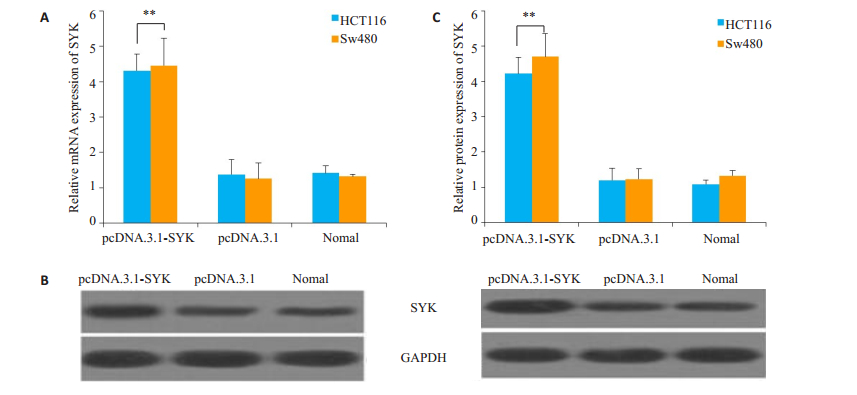
为了检测重组载体转染效率,我们检测了细胞转染后SYK的mRNA和蛋白表达量。结果显示,转染pcDNA.3.1-SYK的实验组(pcDNA.3.1-SYK)中SYK的mRNA和蛋白表达量较转染pcDNA.3.1的阴性对照组(pcDNA.3.1)上调,差异有统计学意义(mRNA HCT116细胞P=0.001,Sw480细胞P=0.004;蛋白表达HCT116细胞P=0.001,Sw480细胞P=0.005),表明SYK过表达成功(图 2)。
|
图 2 过表达后SYK表达量的变化 Figure 2 Expression of SYK in HCT116 and Sw480 cells with SYK overexpression. A: mRNA expression of SYK; B: Western blotting of SYK protein in HCT116 (left) and Sw480 (right); C: Quantitative analysis of Western blotting results. **P < 0.01 vs naive HCT116 and SW480 cells. |
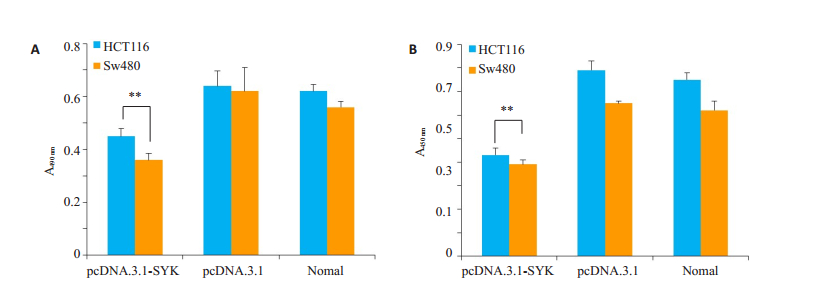
本实验利用MTT方法和BrdU方法检测SYK过表达后,结直肠癌细胞的增殖情况。结果显示,实验组中细胞的生长活力(图 3A)和增值活性(图 3B)显著低于阴性对照组。
|
图 3 过表达SYK后细胞增殖能力的变化 Figure 3 Changes in cell proliferation after SYK overexpression. A: MTT assay; B: BrdU detection; **P < 0.01 vs Negative control group. |
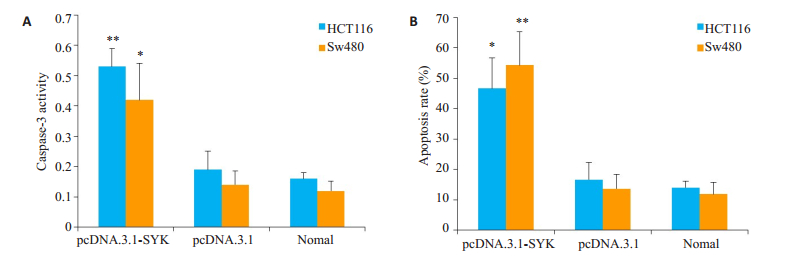
为了检测SYK对结直肠癌细胞凋亡的影响,我们检测了过表达SYK的结直肠癌细胞的caspase-3活性,且利用Annexin-V FITC/PI检测细胞凋亡。结果显示,实验组caspase-3活性和细胞凋亡较阴性对照组升高,差异有统计学意义(caspase-3活性HCT116细胞P= 0.002,Sw480细胞P=0.017;细胞凋亡HCT116细胞P= 0.011,Sw480细胞P=0.004,图 4)。
|
图 4 过表达SYK后细胞caspase-3活性和细胞凋亡变化 Figure 4 Caspase-3 activity (A) and apoptosis (B) of the cells after SYK overexpression. *P < 0.05, **P < 0.01 vs Negative control group. |
为检测在结直肠癌中,SYK过表达是否对Fra-1的表达量产生影响,本实验还检测了Fra-1的mRNA和蛋白表达量。结果显示,实验组Fra-1的mRNA和蛋白质表达量均显著下降,差异具有统计学意义(mRNA HCT116细胞P=0.003,Sw480细胞P=0.003;蛋白表达HCT116细胞P=0.006,Sw480细胞P=0.007,图 5)。
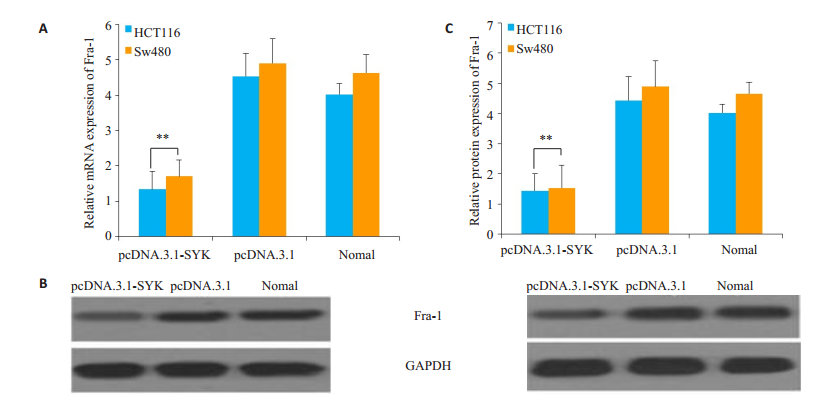
|
图 5 过表达SYK后Fra-1表达量的变化 Figure 5 Changes of Fra-1 expression in HCT116 and SW480 cells after SYK overexpression. A: mRNA expression changes of Fra-1; B: Western blotting of Fra-1 in HCT116 (left) and SW480 (right) cells; C: Quantitative analysis of Western blotting results. **P < 0.01 vs Negative control group. |
结直肠癌是威胁人类健康的主要疾病之一,在恶性肿瘤中死亡率居第2位,占恶性肿瘤发病率的10%及癌症死亡率的8%,并且其发病率及病死率呈上升趋势,危及人类生存[12]。蛋白酪氨酸激酶家族是一组能催化底物蛋白酪氨酸残基磷酸化的酶分子,调控细胞侵袭、增殖过程[13-15]。作为一种非受体型蛋白酪氨酸激酶的SYK同样与细胞增殖等有关,也被认为是一种肿瘤抑制因子[16-17]。
SYK基因在造血细胞中广泛表达,是B细胞受体效应器,可以调控B细胞的克隆扩增、增殖和凋亡[18]。有研究表明,SYK与鳞状细胞癌中细胞的迁移有关[19]。在乳腺癌中SYK的表达量降低,并且SYK具有抑制乳腺癌细胞的生长和转移的作用[7]。SYK是治疗前列腺癌的潜在靶标[20]。SYK可以降低肺癌和胰腺癌细胞的侵袭[13]。Shin等[21]发现在肝细胞癌中,SYK通过抑制肿瘤细胞的增殖、侵袭来行使其肿瘤抑制因子的职能。另外,抑制SYK也有抑制某些肿瘤生长的作用如:抑制SYK可以加强紫杉醇诱导的卵巢癌细胞毒性,有助于卵巢癌疾病的治疗[22]。SYK的抑制可有效治疗高危前体B细胞急性淋巴细胞白血病[23]。然而,对于SYK表达量对结直肠癌细胞增殖和凋亡的研究至今尚未报道。本研究检测到结直肠癌组织和细胞中SYK的表达量均显著下降,另外过表达SYK后,结直肠癌细胞的细胞生长活力降低,细胞凋亡显著上升,说明SYK表达量上调可以抑制结直肠癌细胞的增殖,并且促进其凋亡。
Caspase-3又称半胱氨酸蛋白酶,被称谓死亡蛋白酶,是哺乳动物凋亡的关键蛋白酶[24-25]。Caspase-3的活化后引起一系列下游反应,影响很多重要蛋白质的生理功能,进而促进细胞的凋亡[26-28]。本研究中过表达结直肠癌细胞中的SYK,caspase-3的活性显著升高,促进结直肠癌细胞凋亡。原癌基因Fra-1是核转录因子AP-1家族中Fos亚家族的成员之一[29]。据文献报道,SYK可激活PI3K,但具体机制还不清楚[30-31]。Cao等[32]发现血管平滑肌细胞中PI3K可通过调控AKT来调节Fra-1的表达量,因此SYK对Fra-1的表达量有调控作用。Fra-1作为原癌基因与细胞的增殖、分化、凋亡和肿瘤转化过程密切相关[33-34]。Fra-1可以保护肿瘤细胞免受凋亡,Fra-1表达量减少可促进癌细胞的凋亡[35]。有研究表明,Fra-1表达量的降低可以抑制结肠癌细胞的迁移、侵袭和增殖[36],并且在肺癌中Fra-1表达量的上调抑制癌细胞的凋亡[34]。因此,Fra-1表达量的下调促进肿瘤细胞的凋亡。在本研究中,SYK过表达可以抑制Fra-1的表达,且结直肠癌细胞的增殖能力和凋亡水平分别降低和上升。综上所述,SYK过表达后对结直肠癌细胞增殖能力和凋亡水平的影响可以通过其对Fra-1的调控来实现。
综上,在结直肠癌组织和细胞中,SYK表达量显著降低,过表达SYK可抑制结直癌细胞增殖并促进其凋亡。另外,过表达SYK可以抑制促癌基因Fra-1的表达。本研究表明SYK可作为结直肠癌的抑癌基因,为结直肠癌的治疗提供了一个可能的靶标分子。
| [1] | Vargas AJ, Thompson PA. Diet and nutrient factors in colorectal cancer risk[J]. Nutr Clin Pract, 2012, 27(5): 613-23. DOI: 10.1177/0884533612454885. |
| [2] | Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal Cancer and death[J]. N Engl J Med, 2014, 370(14): 1298-306. DOI: 10.1056/NEJMoa1309086. |
| [3] | Kaminski MF, Wieszczy P, Rupinski MA, et al. Increased rate of adenoma detection associates with reduced risk of colorectal cancer and death[J]. Gastroenterology, 2017, 153(1): 98-105. DOI: 10.1053/j.gastro.2017.04.006. |
| [4] | Kim ER, Chang DK. Colorectal Cancer in inflammatory bowel disease: The risk, pathogenesis, prevention and diagnosis[J]. World J Gastroenterol, 2014, 20(29): 9872-81. DOI: 10.3748/wjg.v20.i29.9872. |
| [5] | Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012[J]. Int J Cancer, 2015, 136(5): E359-86. DOI: 10.1002/ijc.29210. |
| [6] | Kontovounisios C, Kinross J, Tan E, et al. Complete mesocolic excision in colorectal cancer: a systematic review[J]. Colorectal Dis, 2015, 17(1): 7-16. DOI: 10.1111/codi.12793. |
| [7] | Blancato J, Graves A, Rashidi B, et al. SYK allelic loss and the role of Syk-Regulated genes in breast cancer survival[J]. PLoS One, 2014, 9(2): e87610. DOI: 10.1371/journal.pone.0087610. |
| [8] | Naldi A, Larive RM, Czerwinska UA, et al. Reconstruction and signal propagation analysis of the Syk signaling network in breast cancer cells[J]. PLoS Comput Biol, 2017, 13(3): e1005432. DOI: 10.1371/journal.pcbi.1005432. |
| [9] | Krisenko MO, Cartagena A, Raman AA. Nanomechanical property Maps of breast cancer cells as determined by multiharmonic atomic force microscopy reveal Syk-Dependent changes in microtubule stability mediated by MAP1B[J]. Biochemistry, 2015, 54(1): 60-8. DOI: 10.1021/bi500325n. |
| [10] | Koerber RM, Held SA, Heine A, et al. Analysis of the antiproliferative and the pro-apoptotic efficacy of Syk inhibition in multiple myeloma[J]. Exp Hematol Oncol, 2015, 21(4): 21. |
| [11] | Nakashima H, Natsugoe S, Ishigami S, et al. Clinical significance of nuclear expression of spleen tyrosine kinase(Syk)in gastric cancer[J]. Cancer Lett, 2006, 236(1): 89-94. DOI: 10.1016/j.canlet.2005.05.022. |
| [12] | Wong MC, Ching JY, Chan VC, et al. Validation of a risk prediction score for proximal neoplasia in colorectal cancer screening: a prospective colonoscopy study[J]. Sci Rep, 2016, 6(2): 20396. |
| [13] | Peng CL, Zhang Y, Sun QF, et al. Inhibitory effects of Syk transfection on lung cancer cell invasion[J]. Asian Pac J Cancer Prev, 2013, 14(5): 3001-3. DOI: 10.7314/APJCP.2013.14.5.3001. |
| [14] | Lee ST, Feng M, Wei Y, et al. Protein tyrosine phosphatase UBASH3B is overexpressed in triple-negative breast cancer and promotes invasion and metastasis[J]. Proc Natl Acad Sci U S A, 2013, 110(27): 11121-6. DOI: 10.1073/pnas.1300873110. |
| [15] | Shim HJ, Kim HI, Lee ST. The associated pyrazolopyrimidines PP1 and PP2 inhibit protein tyrosine kinase 6 activity and suppress breast cancer cell proliferation[J]. Oncol Lett, 2017, 13(3): 1463-9. |
| [16] | Krisenko MO, Geahlen RL. Calling in SYK: SYK's dual role as a tumor promoter and tumor suppressor in cancer[J]. Biochim Biophys Acta, 2015, 1853(1): 254-63. DOI: 10.1016/j.bbamcr.2014.10.022. |
| [17] | Lindau A, Haerdtner C, Hergeth SP, et al. Atheroprotection through SYK inhibition fails in established disease when local macrophage proliferation dominates lesion progression[J]. Basic Res Cardiol, 2016, 111(2): 20. DOI: 10.1007/s00395-016-0535-8. |
| [18] | Stewart ZA, Pietenpol JA. Syk:a new player in the field of breast cancer[J]. Breast Cancer Res, 2001, 3(1): 5-7. |
| [19] | Luangdilok S, Box C, Patterson L, et al. Syk tyrosine kinase is linked to cell motility and progression in squamous cell carcinomas of the head and neck[J]. Cancer Res, 2007, 67(16): 7907-16. DOI: 10.1158/0008-5472.CAN-07-0331. |
| [20] | Ghotra VP, He S, van der Horst GA, et al. SYK is a candidate kinase target for the treatment of advanced prostate cancer[J]. Cancer Res, 2015, 75(1): 230-40. DOI: 10.1158/0008-5472.CAN-14-0629. |
| [21] | Shin SH, Lee KH, Kim BH, et al. Downregulation of spleen tyrosine kinase in hepatocellular carcinoma by promoter CpG island hypermethylation and its potential role in carcinogenesis[J]. Lab Invest, 2014, 94(12): 1396-405. DOI: 10.1038/labinvest.2014.118. |
| [22] | Yu Y, Gaillard S, Phillip JM, et al. Inhibition of spleen tyrosine kinase potentiates Paclitaxel-Induced cytotoxicity in ovarian cancer cells by stabilizing microtubules[J]. Cancer Cell, 2015, 28(1): 82-96. DOI: 10.1016/j.ccell.2015.05.009. |
| [23] | Burke RT, Meadows S, Loriaux MM, et al. A potential therapeutic strategy for chronic lymphocytic leukemia by combining Idelalisib and GS-9973, a novel spleen tyrosine kinase (Syk) inhibitor[J]. Oncotarget, 2014, 5(4): 908-15. DOI: 10.18632/oncotarget.v5i4. |
| [24] | Zhang Y, Cook A, Kim J, et al. Melatonin inhibits the caspase-1/ cytochrome c/caspase-3 cell death pathway, inhibits MT1 receptor loss and delays disease progression in a mouse model of amyotrophic lateral sclerosis[J]. Neurobiol Dis, 2013, 55(3): 26-35. |
| [25] | Maellaro E, Leoncini S, Moretti D, et al. Erythrocyte caspase-3 activation and oxidative imbalance in erythrocytes and in plasma of type 2 diabetic patients[J]. Acta Diabetol, 2013, 50(4): 489-95. DOI: 10.1007/s00592-011-0274-0. |
| [26] | Brentnall M, Rodriguez-Menocal L, De Guevara RL, et al. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis[J]. BMC Cell Biol, 2013, 14(5): 32. |
| [27] | Khalil H, Bertrand MJ, Vandenabeele P, et al. Caspase-3 and RasGAP: a stress-sensing survival/demise switch[J]. Trends Cell Biol, 2014, 24(2): 83-9. DOI: 10.1016/j.tcb.2013.08.002. |
| [28] | Wu JH, Yao YL, Gu T, et al. MiR-421 regulates apoptosis of BGC-823 gastric cancer cells by targeting caspase-3[J]. Asian Pac J Cancer Prev, 2014, 15(13): 5463-8. DOI: 10.7314/APJCP.2014.15.13.5463. |
| [29] | Hasenfuss SC, Bakiri L, Thomsen MK, et al. Activator protein 1 transcription factor fos-related antigen 1(Fra-1)is dispensable for murine liver fibrosis, but modulates xenobiotic metabolism[J]. Hepatology, 2014, 59(1): 261-73. DOI: 10.1002/hep.26518. |
| [30] | Jiang K, Zhong B, Gilvary DL, et al. Syk regulation of phosphoinositide 3-kinase-dependent NK cell function[J]. J Immunol, 2002, 168(7): 3155-64. DOI: 10.4049/jimmunol.168.7.3155. |
| [31] | Becker KL, Aimanianda V, Wang X, et al. Aspergillus cell wall chitin induces anti-and proinflammatory cytokines in human PBMCs via the fc-gamma receptor/Syk/PI3K pathway[J]. MBio, 2016, 7(3): e01815-23. |
| [32] | Cao H, Dronadula N, Rao GN. Thrombin induces expression of FGF-2 via activation of PI3K-Akt-Fra-1 signaling axis leading to DNA synthesis and motility in vascular smooth muscle cells[J]. Am J Physiol Cell Physiol, 2006, 290(1): C172-82. DOI: 10.1152/ajpcell.00284.2005. |
| [33] | Oliveira-Ferrer L, Kuerschner M, Labitzky V, et al. Prognostic impact of transcription factor Fra-1 in ER-positive breast cancer: contribution to a metastatic phenotype through modulation of tumor cell adhesive properties[J]. J Cancer Res Clin Oncol, 2015, 141(10): 1715-26. DOI: 10.1007/s00432-015-1925-2. |
| [34] | Zhong GW, Chen X, Fang X, et al. Fra-1 is upregulated in lung cancer tissues and inhibits the apoptosis of lung cancer cells by the P53 signaling pathway[J]. Oncol Rep, 2016, 35(1): 447-53. DOI: 10.3892/or.2015.4395. |
| [35] | Verde P, Casalino L, Talotta F, et al. Deciphering AP-1 function in tumorigenesis: fra-ternizing on target promoters[J]. Cell Cycle, 2007, 6(21): 2633-9. DOI: 10.4161/cc.6.21.4850. |
| [36] | Wu JM, Wu G, Lv L, et al. MicroRNA-34a inhibits migration and invasion of colon cancer cells via targeting to Fra-1[J]. Carcinogenesis, 2012, 33(3): 519-28. DOI: 10.1093/carcin/bgr304. |
 2017, Vol. 37
2017, Vol. 37

