2. 西安交通大学医学部 环境与疾病相关基因教育部重点实验室,陕西 西安 710061
2. Key Laboratory of Environment and Genes Related to Diseases, School of Basic Medical Sciences, Xi'an Jiaotong University Health Science Centre, Xi'an710061, China
转运蛋白ATP-盒式结合转运子-A1 (ABCA1)是在介导胆固醇反向转运中发挥核心作用的跨膜蛋白,通过直接与载脂蛋白ApoA-Ⅰ和ApoE结合,介导胆固醇向载脂蛋白组分转运,其缺失能够阻断绝大部分胆固醇反向转运[1-2]。近几年,有研究显示ABCA1可通过与HDL介导的胆固醇反向转运作用抑制炎症反应[3-6],还可通过参与细胞胆固醇和磷脂转运、细胞表面脂质结构域的形成等多个环节对炎症进行抑制性调节[7-12]。当ABCA1缺失条件下,小鼠腹腔巨噬细胞受到TLR2、3和4的配体刺激,相关的炎性细胞因子和趋化因子表达能发生显著上调[11, 13-14]。
ATF3 (活化转录因子3)是转录活化因子/cAMP反应元件结合蛋白(ATF/CREB)转录因子家族成员,通过自身亮氨酸拉链结构与其他有相同结构域的转录因子同二聚化或异二聚化结合于炎症相关因子启动子区,抑制其转录活化,调节多个炎症相关因子的表达,是重要的炎症抑制因子[15-16]。ATF3在静息期维持低表达水平,而内质网压力应激等条件下表达上调并发挥抗炎作用[17-19]。有研究提出高胆固醇饲养能够上调小鼠体内ATF3表达并激活对Th细胞中ATF3依赖性的免疫抑制作用[20]。而在ABCA1通过胆固醇转运和膜表面脂质结构域形成等多种机制对炎症进行抑制性调节的进程中,是否参与了对抑制性转录因子ATF3的表达调节尚不清楚。为了检测ABCA1在参与炎症调节中是否参与ATF3的转录调节,本文构建了小鼠单核巨噬细胞RAW264.7的ABCA1敲低细胞系、利用TLR2配体Pam3CSK4刺激该细胞系建立炎症反应细胞模型、检测促炎细胞因子IL-1β、TNF-α和IL-6在ABCA1敲低的RAW264.7重组细胞系中的表达变化以及ATF3及其家族成员的表达变化。
1 材料和方法 1.1 材料MSH030800-7-CU6 shRNA质粒(复能生物构建),Fugene HD转染试剂购自Promega,1640培养基购自Hyclone,胎牛血清购自BI;RAW264.7小鼠单核巨噬细胞系购自中科院上海细胞库。ABCA1小鼠单抗购自Abcam,ATF3兔多抗购自SANTA,β-actin兔单抗购自CST。IgG山羊抗鼠多抗以及IgG山羊抗兔多抗购自昕泰生物,Western及IP细胞裂解液,SDS-PAGE凝胶配制试剂盒购自碧云天,TriPure Isolation Reagent和FastStart Universal SYBR Green Master (ROX)购自Roche,First Strand cDNA Synthesis Kit购自Thermo,其余试剂均为进口分析纯。
1.2 方法 1.2.1 ABCA1 KD重组细胞系构建查询NCBI数据库,选择编号为Pr006106100的siRNA序列>Probe| 6106100|SIRNA_SENSE siRNA sense sequence (21b) GGATGTATAATGAGCAGTATT>Probe|6106100|SIR -NA_ANTISENSE siRNA antisense sequence (21b)TAC TGCTCATTATACATCCTT,另委托复能公司预测3处ABCA1 KD位点,设计3对备用siRNA序列,应用以上4对siRNA序列,以复能公司的shRNA载体psi-U6TM作为载体骨架[21],委托复能公司构建shRNA载体,共计4份,分别记为:ABCA1sh1、ABCA1 sh2、ABCA1sh3和ABCA1sh4;另外对照shRNA载体记为NC。
复苏1支RAW264.7细胞,以10%胎牛血清的1640培养基10 mL,培养于直径10 cm的平皿中,5% CO2,37 ℃恒温培养,隔天传代。取传代3次的RAW264.7细胞,每孔2×106种植于6孔板,共计种6孔,培养过夜。将5份质粒分别转入5孔细胞,每种质粒取3.3 μg与153 μL无血清1640培养基混匀,再加入13.5 μL Fugene HD转染试剂,吹打混匀,静置包被10~15 min。吸取混匀后的质粒包被混合物,缓慢滴加入孔,每孔150 μL,在超净台上平行摇晃混匀,37 ℃恒温培养24 h后换液,每孔细胞加嘌呤霉素(终浓度为3 μg/mL)用于筛选ABCA1敲低(ABCA1 KD)的稳定细胞系,其中1孔未做转染的细胞作为空白对照。37 ℃,5% CO2条件下培养,隔天以含3 μg/mL嘌呤霉素的1640培养基进行换液。15 d内对照孔细胞全部死亡后,对转染孔新增殖细胞分别进行扩增培养并冻存。所得到稳定抗性细胞系分别标记为RAW264.7-ABCA1NC,RAW264.7-ABCA1sh1,RAW 264.7-ABCA1sh2,RAW264.7-ABCA1sh3和RAW264.7-ABCA1sh4。
1.2.2 ABCA1 KD稳定抗性细胞系鉴定TriPure Isolation Reagent提取RAW264.7-ABCA1NC,RAW 264.7-ABCA1sh1,RAW264.7-ABCA1sh2,RAW264.7-ABCA1sh3和RAW264.7-ABCA1sh4稳定抗性细胞系总RNA。将RAW264.7-ABCA1NC,RAW264.7-ABCA1sh1,RAW264.7-ABCA1sh2,RAW264.7-ABCA1sh3和RAW264.7-ABCA1sh4稳定抗性细胞系共计5株分别以每孔2×106种植于6孔板,每株种3个平行副孔,共计15孔,以含3 μg/mL嘌呤霉素的1640培养基培养24 h后,吸去培养基上清,提取细胞系总RNA,检测ABCA1转录水平在稳定抗性细胞系中的变化。
将RAW264.7-ABCA1NC,RAW264.7-ABCA1sh1稳定抗性细胞系共计2株,分别以每孔2×106种植于6孔板,每株细胞系种3个平行副孔,共计6孔,以含3 μg/mL嘌呤霉素的1640培养基培养24 h后,吸去培养基上清,提取细胞系总蛋白,检测ABCA1蛋白表达水平在稳定抗性细胞系中的变化。
1.2.3 Pam3CSK4对RAW264.7的ABCA1 KD细胞株的刺激将RAW264.7-ABCA1NC,和RAW264.7-ABCA1sh1分别种24孔板,每孔5×105细胞,两株重组细胞系各种6孔,培养过夜,标记为NC和sh1.以终浓度为100 ng/mL的Pam3CSK4分别对NC和sh1重组细胞系进行刺激,刺激孔设置为3孔,对照组加入等量PBS,设置为3孔,刺激6h后,Tripure法提取细胞总RNA,实时定量荧光PCR检测各组细胞中IL-1β、IL-6、TNF-α、ATF1、ATF2、ATF3、ATF4和ATF5在转录水平的表达差异。
将RAW264.7-ABCA1NC,和RAW264.7-ABCA1sh1分别种6孔板,每孔2×106细胞,两株重组细胞系各种6孔,培养过夜,标记为NC和sh1。以终浓度为100 ng/mL的Pam3CSK4分别对NC和sh1重组细胞系进行刺激,刺激孔设置为3孔,对照组加入等量PBS,设置为3孔,刺激24 h后,提取细胞总蛋白,Western blotting检测各组细胞中ATF3在蛋白水平的表达差异。
1.2.4 总RNA的提取TriPure Isolation Reagent提取细胞系总RNA。每孔加入1 mL TriPure,将细胞裂解并收入1.5 mL Ep管中。每管TriPure样品加入CHCl3 200 μL,颠倒震荡混匀,静置5 min,4 ℃,12 000 g离心15 min,吸取450 μL上清到新的干净的Ep管中,加入450 μL异丙醇,颠倒震荡混匀,-20 ℃静置过夜,12 000 g离心15 min,吸去上清,加入1 mL 75%乙醇,颠倒震荡混匀,12 000 g离心15 min,尽量吸去上清,开盖静置至乙醇完全挥发,每管加入DEPC水10 μL,使RNA沉淀完全溶解。
1.2.5 cDNA的反转录对样品定量后,取5 μg总RNA进行反转录。反转录程序为,总RNA 5 μg,mRNA反转录通用引物Oligo (dT) 1 μL,与DEPC水混合成总体积12 μL的反应体系,该反应体系在65 ℃条件下孵育5 min后,冰浴条件下加入2 μL dNTP Mix (10 mmol/L),4 μL 5 × Reaction Buffer,1 μL RiboLock RNase Inhibitor (20 U/μL)和1 μL RevertAid M-MuLV RT (200 U/μL),反应总体积为20 μL,温和混匀所有组分并离心后,42 ℃反应60 min,最终72 ℃恒温5 min以终止反应,该反应完成后扩增得到样品总cDNA。
1.2.6 实时荧光定量PCR荧光实时定量PCR检测目的基因的转录水平。将样品总cDNA稀释20倍作为荧光实时定量PCR扩增模板。扩增体系为,cDNA模板4 μL,上下游引物各1 μL,FastStart Universal SYBR Green Master (ROX) 5 μL,于Bio-Rad iQ5荧光定量扩增PCR仪反应40个循环,检测ABCA1基因的转录水平。
1.2.7 总蛋白的提取吸去细胞种植孔中培养基上清,每孔加入1 mL PBS冲洗后,小心吸去,再次每孔加入1 mL PBS,用细胞刮小心地将细胞刮下,将细胞混悬液移入干净Ep管中,4 ℃,3000 r/min离心15 min,去除上清,每管加入含PMSF 1 mmol/L的Western及IP细胞裂解液100 μL,混匀,冰浴静置30 min,4 ℃,13 000 r/min离心15 min,收取上清转入新的干净Ep管中,定量后将蛋白稀释至终浓度1.5 μg/μL,加入蛋白上样变性缓冲液,100 ℃,10 min变性。
1.2.8 Western blotting(1) ABCA1 Western blotting
制备SDS-PAGE凝胶,浓缩胶浓度为5%,分离胶浓度为6%,每孔内上样总蛋白30 μg,80 V恒压30 min后,120 V恒压2.5 h,共计电泳3 h。裁孔径大小为0.45 μm的NC膜,从阳极到阴极制备顺序为“滤纸-NC膜-胶-滤纸”的“三明治”结构,冰浴条件下,320 mA恒流转印2.5 h。转印后将NC膜于5%脱脂奶粉-TBST溶液中,室温条件下,80 r/min震荡封闭3 h。
将2 μL ABCA1小鼠单抗以1:1000体积比稀释于2 mL 5%脱脂奶粉-TBST溶液中,覆盖于蛋白印迹上,4 ℃孵育过夜;TBST缓冲液震荡洗膜3次,每次15 min。将1 μL羊抗小鼠IgG以1:5000体积比稀释于5 mL 5%脱脂奶粉-TBST溶液中,覆盖于蛋白印迹上,室温条件下,80 r/min震荡孵育2 h。TBST缓冲液震荡洗膜3次,每次15 min,TBS缓冲液洗膜15 min。取ECL超强化学发光显影液500 μL,反应缓冲液500 μL,配制化学发光显影工作液1 mL,将配制好的化学发光显影工作液滴加于NC膜蛋白印迹上,凝胶成像系统曝光8 min拍照成像。
(2) 其他蛋白的Western blotting
制备SDS-PAGE凝胶,浓缩胶浓度为5%,分离胶浓度为10%,每孔内上样总蛋白30 μg,80 V恒压30 min后,120 V恒压2.5 h,共计电泳3 h。裁孔径大小为0.22 μm的PVDF膜,从阳极到阴极制备顺序为“滤纸-PVDF膜-胶-滤纸”的“三明治”结构,15 V恒压转印45 min。转印后将PVDF膜于5%脱脂奶粉-TBST溶液中,室温条件下,80 r/min震荡封闭3 h。
将20 μL ATF3兔多抗以1:150体积比稀释于3 mL 5%脱脂奶粉-TBST溶液中,2 μLβ-Actin兔单抗以1:1000体积比稀释于2 mL 5%脱脂奶粉-TBST溶液中,覆盖于蛋白印迹上,4 ℃孵育过夜;TBST缓冲液震荡洗膜3次,每次15 min。将1 μL羊抗兔IgG以1:5000体积比稀释于5 mL 5%脱脂奶粉-TBST溶液中,覆盖于蛋白印迹上,室温条件下,80 r/min震荡孵育2 h。TBST缓冲液震荡洗膜3次,每次15 min,TBS缓冲液洗膜15 min。取ECL超强化学发光显影液500 μL,反应缓冲液500 μL,配制化学发光显影工作液1 mL,将配制好的化学发光显影工作液滴加于PVDF膜蛋白印迹上,凝胶成像系统曝光2~8 min拍照成像。
1.2.9 数据统计所有实验均重复3遍,数据采用Prism 6.0软件统计,统计方法为t检验,P<0.05为差异有统计学意义。
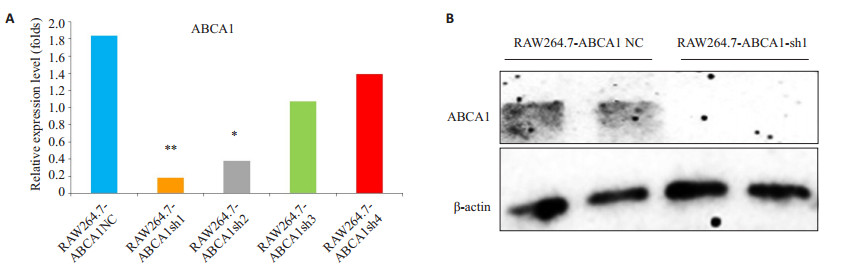
2 结果 2.1 ABCA1 KD稳定抗性细胞系的鉴定实时定量荧光PCR结果显示,与RAW264.7-ABCA1NC相比较,4种shRNA稳定抗性细胞系中,RAW264.7-ABCA1sh1中ABCA1在RNA水平相对表达量最低,符合对ABCA1 KD的要求(图 1 A,P<0.01)。与RAW264.7-ABCA1NC相比,RAW264.7-ABCA1sh2中ABCA1在RNA水平的相对表达量也有显著降低(图 1A,P<0.05)。我们选择RAW264.7-ABCA1sh1稳定细胞株,进一步鉴定shRNA质粒是否能够在蛋白质水平对ABCA1的进行敲低。
|
图 1 ABCA1 KD稳定抗性细胞系的鉴定 Figure 1 Identification of RAW264.7 cell line with stable ABCA1 knockdown. A: Relative expression level of ABCA1 in different cell lines; B: Relative proteinlevel of ABCA1 in NC and cells with ABCA1 knockdown (ABCA1-sh1). *P < 0.05, **P < 0.01 vs RAW264.7-ABCA1NC. |
Western blotting显示,与RAW264.7-ABCA1NC相比较,RAW264.7-ABCA1sh1中ABCA1在蛋白水平的表达下调(图 1B)。RNA水平和蛋白质水平的鉴定结果显示,在构建的4株RAW264.7的ABCA1 KD稳定细胞系中,RAW264.7-ABCA1sh1细胞株的ABCA1的mRNA和蛋白质表达水平均下调,因此,选择RAW264.7-ABCA1sh1细胞株用于后续的实验研究。
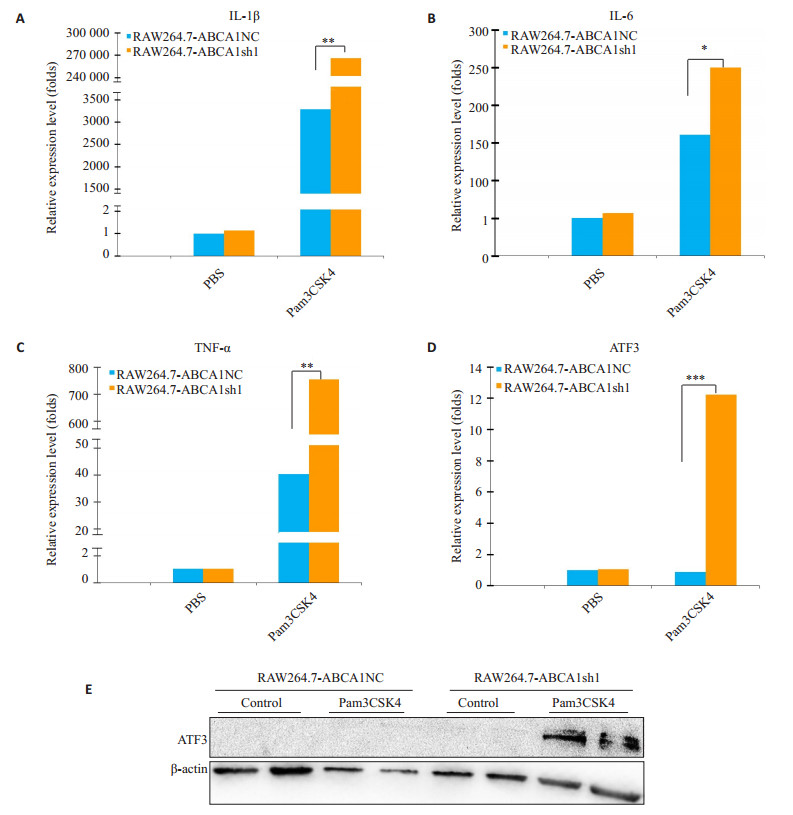
2.2 ABCA1 KD细胞系中Pam3CSK4刺激后,炎症相关基因的表达变化实时定量PCR统计结果显示,Pam3CSK4刺激6 h后,RAW264.7-ABCA1NC组中TNF-α,IL-1β和IL-6的表达均较PBS处理组发生了上调,转录抑制因子ATF3的表达没有发生显著变化(图 2A~2D)。同样在Pam3CSK4刺激后,与RAW264.7-ABCA1NC组比较,RAW264.7-ABCA1sh1组中TNF-α,IL-1β和IL-6表达发生显著上调,而炎症抑制转录因子ATF3表达也发生了显著上调(图 2A~2D,P<0.05)。Western结果显示,RAW264.7-ABCA1sh1组细胞系在被Pam3CSK4刺激24 h后,ATF3的蛋白表达也发生上调,而RAW264.7-ABCA1NC组细胞系在被Pam3CSK4刺激24 h后,ATF3表达未发生变化(图 2E)。
|
图 2 ABCA1 KD重组细胞系中Pam3CSK4刺激后,促炎和抑炎相关基因的表达 Figure 2 Relative expression of pro-inflammatory and anti-inflammatory genes in response to Pam3CSK4 stimulation in RAW264.7 cell line with stable ABCA1 knockdown. A-D: Relative expression levels of IL-1β, IL-6, TNF-α, and ATF3 mRNAs, respectively; E: Western blotting for ATF3 expressions. *P < 0.05, **P < 0.01, ***P < 0.001 vs PBS group. |
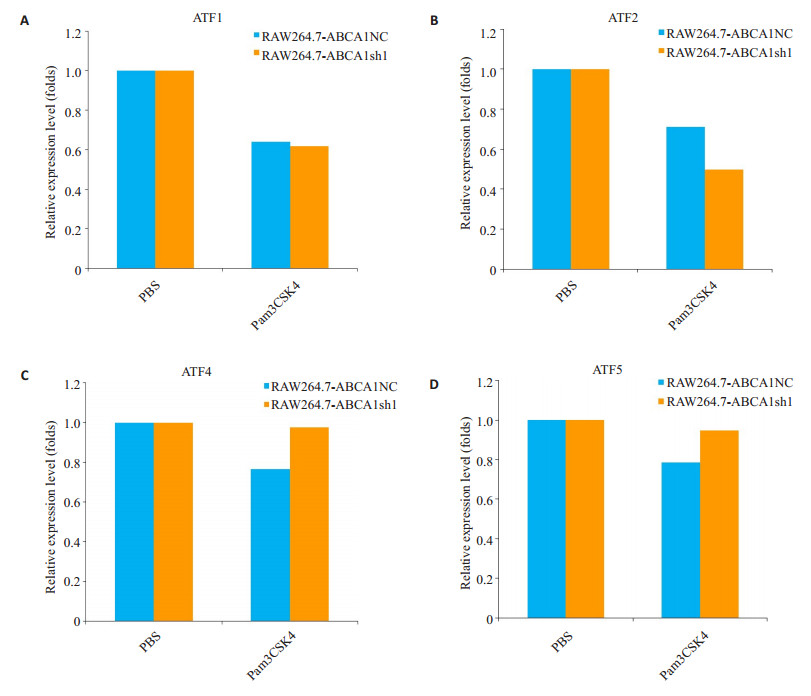
实时定量PCR统计结果显示,Pam3CSK4刺激6 h后与PBS处理组相比较,ATF1和ATF2在RAW264.7-ABCA1NC组和RAW264.7-ABCA1sh1组的转录水平均下调,但受到Pam3CSK4刺激后,RAW264.7-ABCA1NC组和RAW264.7-ABCA1sh1组间ATF1和ATF2的转录则均无显著差异(P>0.05,图 3A和图 3B),受到Pam3CSK4刺激6 h后,ATF4和ATF5在RAW264.7-ABCA1NC组和RAW264.7-ABCA1sh1组的转录水平与PBS处理组相比较,没有发生显著变化(图 3C、D,P>0.05)。
|
图 3 ABCA1 KD重组细胞系中Pam3CSK4刺激后,ATF家族其他基因的表达 Figure 3 Relative expression of the other members of ATFs familyin RAW264.7 cell line with stable ABCA1 knockdown stimulated with Pam3CSK4. A-D: Expression levels of ATF1, ATF2, ATF4, and ATF5, respectively. |
ABCA1能够通过胆固醇转运和影响细胞表面脂质结构域减少MyD88依赖的促炎细胞因子如TNF-α、IL-1β、IL-6和IL-12p40的表达[22],在巨噬细胞中特异性敲除ABCA1能够引起胆固醇的大量累积,上调这些细胞因子的表达,进而在动脉粥样硬化进程中引发斑块炎症[23-24]。与此相对应,也有研究报道称,在高脂饮食喂养并发生胰岛素抵抗的肥胖小鼠中,特异性敲除髓细胞ABCA1不能加重脂肪组织中的炎症反应[25]。因此,ABCA1对炎症的调节方向还有待进一步发现。
我们在工作中应用shRNA载体,对RAW264.7进行转染,以嘌呤霉素筛选得到稳定抗性细胞系。经过实时定量PCR和Western蛋白印迹检测,确定了ABCA1被稳定敲低的RAW264.7重组细胞株,编号RAW264.7-ABCA1 sh1。我们应用ABCA1被稳定敲低的RAW264.7重组细胞株,包含了作为对照的RAW264.7-ABCA1 NC和ABCA1被敲低的RAW264.7-ABCA1 sh1,对两组细胞株应用Pam3CSK4进行刺激,首先发现,相比于未受刺激的PBS处理组,Pam3CSK4刺激组中,促炎因子IL-1β、IL-6和TNF-α的转录表达发生显著上调。在受到Pam3CSK4刺激的细胞系中,与RAW264.7-ABCA1 NC组相比,RAW264.7-ABCA1 sh1组经Pam3CSK4刺激后IL-1β、IL-6和TNF-α的转录水平上调幅度更高,提示ABCA1敲除会显著增强Pam3CSK4刺激引起的炎症相关细胞因子表达上调,这与文献报道的敲低ABCA1会引起炎症相关细胞因子表达上调的结论一致[23-24],验证了ABCA1在炎症抑制中发挥着重要作用。
De Nardo等[26]在研究中发现,新生HDL作用于小鼠巨噬细胞能够上调ATF3的转录,于是我们提出作为新生HDL胆固醇RCT关键受体的ABCA1可能参与了ATF3的转录调节。为了验证ABCA1是否对ATF3的转录有调节作用,我们在所构建的ABCA1 KD炎症细胞模型中检测了ATF3及其蛋白家族其它成员的转录水平变化。在应用shRNA构建了RAW264.7的ABCA1稳定敲低细胞系之后,对敲低细胞系以Pam3CSK4进行刺激,比对敲低组和对照组之间,炎症细胞因子的表达变化。结果发现,RAW264.7-ABCA1NC受到Pam3CSK4刺激后,IL-1β等促炎细胞因子转录发生上调的同时,细胞内ATF3的转录水平没有发生显著变化;而RAW264.7-ABCA1sh1组受Pam3CSK4刺激后,在IL-1β等促炎细胞因子转录上调进一步增强的同时,ATF3的转录水平发生了显著上调。但是,同样RAW264.7-ABCA1sh1组,受Pam3CSK4刺激的条件下,ATF3所在转录因子家族的其他成员ATF1、ATF2、ATF4和ATF5的转录均没有发生显著的上调变化;这些结果说明,在Pam3CSK4刺激巨噬细胞株RAW264.7所引起炎症效应中,ABCA1敲低不但可以增强促炎细胞因子的表达,而且还可以引起炎症抑制性转录因子ATF3的表达上调,对ATF3的上调作用在ATF家族其它蛋白因子中并未发生。这样的结果提示了ABCA1对Pam3CSK4刺激所引起的炎症效应的调节有可能是双向的,且其对ATF3的转录上调的调节机制具有特异性,该特异性机制尚有待进一步揭示和阐明。
| [1] | Du XM, Kim MJ, Hou L, et al. HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export[J]. Circ Res, 2015, 116(7): 1133-42. DOI: 10.1161/CIRCRESAHA.116.305485. |
| [2] | Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity[J]. Nat Rev Immunol, 2015, 15(2): 104-16. DOI: 10.1038/nri3793. |
| [3] | Khalil A, Berrougui H, Pawelec G, et al. Impairment of the ABCA1 and SR-BI-mediated cholesterol efflux pathways and HDL antiinflammatory activity in Alzheimer's disease[J]. Mech Ageing Dev, 2012, 133(1): 20-9. DOI: 10.1016/j.mad.2011.11.008. |
| [4] | Bi X, Zhu XW, Duong MN, et al. Liver ABCA1 deletion in LDLrKO mice does not impair macrophage reverse cholesterol transport or exacerbate atherogenesis[J]. Arterioscler Thromb Vasc Biol, 2013, 33(10): 2288-96. DOI: 10.1161/ATVBAHA.112.301110. |
| [5] | Wang S, Gulshan K, Brubaker G, et al. ABCA1 mediates unfolding of apolipoprotein AI N terminus on the cell surface before lipidation and release of nascent high-density lipoprotein[J]. Arterioscler Thromb Vasc Biol, 2013, 33(6): 1197-205. DOI: 10.1161/ATVBAHA.112.301195. |
| [6] | Mineo C, Shaul PW. Regulation of signal transduction by HDL[J]. J Lipid Res, 2013, 54(9): 2315-24. DOI: 10.1194/jlr.R039479. |
| [7] | Murphy AJ, Westerterp M, Yvan-Charvet L, et al. Anti-atherogenic mechanisms of high density lipoprotein: effects on myeloid cells[J]. Biochim Biophys Acta, 2012, 1821(3): 513-21. DOI: 10.1016/j.bbalip.2011.08.003. |
| [8] | Westerterp M, Murphy AJ, Wang M, et al. Deficiency of ATPbinding cassette transporters A1 and G1 in macrophages increases inflammation and accelerates atherosclerosis in mice[J]. Circ Res, 2013, 112(11): 1456-65. DOI: 10.1161/CIRCRESAHA.113.301086. |
| [9] | Westerterp M, Bochem AE, Yvan-Charvet L, et al. ATP-binding cassette transporters, atherosclerosis, and inflammation[J]. Circ Res, 2014, 114(1): 157-70. DOI: 10.1161/CIRCRESAHA.114.300738. |
| [10] | Yin K, Chen WJ, Zhou ZG, et al. Apolipoprotein A-Ⅰ inhibits CD40 proinflammatory signaling via ATP-binding cassette transporter A1-mediated modulation of lipid raft in macrophages[J]. J Atheroscler Thromb, 2012, 19(9): 23-35. |
| [11] | Pagler TA, Wang M, Mondal M, et al. Deletion of ABCA1 and ABCG1 impairs macrophage migration because of increased Rac1 signaling[J]. Circ Res, 2011, 108(2): 194-200. DOI: 10.1161/CIRCRESAHA.110.228619. |
| [12] | Chai AB, Ammit AJ, Gelissen IC. Examining the role of ABC lipid transporters in pulmonary lipid homeostasis and inflammation[J]. Respir Res, 2017, 18(1): 1-9. DOI: 10.1186/s12931-016-0492-7. |
| [13] | Suzuki M, Pritchard DK, Becker L, et al. High-density lipoprotein suppresses the type Ⅰ interferon response, a family of potent antiviral immunoregulators, in macrophages challenged with lipopolysaccharide[J]. Circulation, 2010, 122(19): 1919-27. DOI: 10.1161/CIRCULATIONAHA.110.961193. |
| [14] | Frisdal E, Lesnik P, Olivier M, et al. Interleukin-6 protects human macrophages from cellular cholesterol accumulation and attenuates the proinflammatory response[J]. J Biol Chem, 2011, 286(35): 30926-36. DOI: 10.1074/jbc.M111.264325. |
| [15] | Boespflug ND, Kumar S, McAlees JW, et al. ATF3 is a novel regulator of mouse neutrophil migration[J]. Blood, 2014, 123(13): 2084-93. DOI: 10.1182/blood-2013-06-510909. |
| [16] | Zhou H, Guo HP, Zong J, et al. ATF3 regulates multiple targets and may play a dual role in cardiac hypertrophy and injury[J]. Int J Cardiol, 2014, 174(3): 838-9. DOI: 10.1016/j.ijcard.2014.04.160. |
| [17] | Lu D, Chen J, Hai T. The regulation of ATF3 gene expression by mitogen-activated protein kinases[J]. Biochem J, 2007, 401(2): 559-67. DOI: 10.1042/BJ20061081. |
| [18] | Wang Y, Zhang YW, Xu L, et al. The potential role of ATF3 on immune response is regulated by BMP10 through Smad dependent pathway[J]. Med Hypotheses, 2011, 76(5): 685-8. DOI: 10.1016/j.mehy.2011.01.033. |
| [19] | Aung HH, Lame MW, Gohi K, et al. Induction of ATF3 gene network by triglyceride-rich lipoprotein lipolysis products increases vascular apoptosis and inflammation[J]. Arterioscler Thromb Vasc Biol, 2013, 33(9): 2088-96. DOI: 10.1161/ATVBAHA.113.301375. |
| [20] | Liu Y, Tang C. Regulation of ABCA1 functions by signaling pathways[J]. Biochim Biophys Acta, 2012, 1821(3): 522-9. DOI: 10.1016/j.bbalip.2011.08.015. |
| [21] | GeneCopoeia. psi-U6 ubdate 2008[EB/OL]. [2016-12-1]. http://www.fulengen.com/wp-content/uploads/oldpdfs/product/shrna/psiU6.pdf |
| [22] | Zhu X, Owen JS, Wilson MD, et al. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol[J]. J Lipid Res, 2010, 51(11): 3196-206. DOI: 10.1194/jlr.M006486. |
| [23] | Tang C, Liu Y, Kessler PS, et al. The macrophage cholesterol exporter ABCA1 functions as an anti-inflammatory receptor[J]. J Biol Chem, 2009, 284(47): 32336-43. DOI: 10.1074/jbc.M109.047472. |
| [24] | Albers JJ, Vuletic S, Cheung MC. Role of plasma phospholipid transfer protein in lipid and lipoprotein metabolism[J]. Biochim Biophys Acta, 2012, 1821(3): 345-57. DOI: 10.1016/j.bbalip.2011.06.013. |
| [25] | Zhu X, Chung S, Bi X, et al. Myeloid cell-specific ABCA1 deletion does not worsen insulin resistance in HF diet-induced or genetically obese mouse models[J]. J Lipid Res, 2013, 54(10): 2708-17. DOI: 10.1194/jlr.M038943. |
| [26] | De Nardo D, Labzin LI, Kono H, et al. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3[J]. Nat Immunol, 2014, 15(2): 152-63. |
 2017, Vol. 37
2017, Vol. 37

