2. 首都医科大学附属北京朝阳医院西区皮肤科,北京 100043;
3. 西安交通大学第一附属医院泌尿外科,陕西 西安 710061
2. Department of Dermatology and Venereology, Jingxi Campus of Beijing Chaoyang Hospital, Capital Medical University, Beijing 100043, China;
3. Department of Urology, First Affiliated Hospital of Xi'an Jiaotong University, Xi'an 710061, China
银屑病是临床常见的一种慢性复发性的炎症性皮肤病,超过90%的银屑病患者为寻常型银屑病(PV),其组织病理表现主要为表皮角质增厚、真皮乳头层血管增生和炎症细胞浸润[1-3]。然而,银屑病的病因及发病机制尚不完全清楚。存活素(Survivin)作为一种凋亡蛋白抑制因子(IAP),广泛表达于各种肿瘤组织中,如乳腺癌、口腔癌、肺癌和肝癌等[4-7]。然而,目前Survivin在银屑病中的表达水平仍存在争议。磷脂酰肌醇3-激酶(PI3K)/蛋白激酶B(AKT)信号通路是肿瘤研究的热门信号通路,它参与了肿瘤的增殖、转移、耐药以及凋亡抑制等过程[8]。目前研究发现其与银屑病的发生发展可能相关[9-10]。为进一步探索Survivin、PI3K、AKT在银屑病发病机制中的作用,本研究在基因、蛋白、细胞水平并应用小干扰RNA(siRNA)策略,检测人银屑病皮损角质形成细胞(KCs)中Survivin、PI3K和AKT蛋白和mRNA的表达水平并探讨其相关性和意义。
1 材料和方法 1.1 临床资料寻常型银屑病(PV)患者22例,均来自西安交通大学第二附属医院皮肤科门诊患者,患者均具有典型的临床症状及病理特征,符合PV进行期的诊断标准,其中男性12例,女性10例,年龄24~60岁,平均41.68岁;病程3月~22年,平均7.63年。以同期我院皮肤外科18例美容整形患者切除的正常皮肤组织作为对照组,其中男性10例,女性8例,年龄15~51岁,平均39.81岁。所有银屑病患者取材前3月未服用或外用糖皮质激素、免疫抑制剂及维A酸类药物,取材前2周内未使用任何药物治疗,且不伴有其他皮肤及系统疾病。所有标本取材均按常规皮肤活检手术进行。银屑病患者组与健康对照组在年龄、性别等方面比较差异无统计学意义(P>0.05)。
1.2 免疫组织化学采用EnVision二步法:65 ℃烤片,二甲苯脱蜡,酒精梯度水化,高压锅抗原修复5 min,阻断内源性过氧化物酶10 min,一抗(Survivin、PI3K、AKT均购买于北京博奥森生物公司)(浓度参考抗体说明书)孵育4 ℃过夜,二抗常温孵育30 min,DAB显色,苏木素复染,盐酸分化和氨水反蓝,酒精梯度脱水,二甲苯透明和中性树胶封片,最后再显微镜下观察和拍片。免疫组织化学染色评分:根据着色强度(无、弱、中等、强)分别评0、1、2、3分;根据阳性细胞百分比[(0~25)%、(25~50)%、(50~ 75)%、(75~100)%]分别评1、2、3、4分,总分为着色强度评分和阳性细胞百分比评分的乘积。
1.3 细胞培养将HaCaT细胞株培养于含10%胎牛血清的1640培养基(Gibco)中,并将细胞置于37 ℃、5% CO2的培养箱中进行培养。
1.4 siRNA转染AKT siRNA(吉玛生物),siAKT序列为:正义链5'-UUGUAGCCAAUGAAGGUGCCA-3',反义链5'-GC ACCUUCAUUGGCUACAATT-3',转染试剂X-tremeGENEsiRNA transfection reagents购自Roche公司。将1.5×105 HaCaT细胞种植于6孔细胞培养板。24 h后采用X-tremeGENE siRNA transfection reagents将2 µg/孔siRNA转染到细胞内,继续培养48 h后提取细胞总蛋白。
1.5 Western blot常规提取组织和细胞总蛋白,使用10%的SDSPAGE凝胶进行电泳分离蛋白,电泳后采用湿转的方法将蛋白转移至PVDF膜上,5%脱脂牛奶常温封闭1 h,一抗(Survivin、PI3K、AKT和GAPDH均购买于北京博奥森生物公司)孵育4 ℃过夜,TBST洗涤3次,5 min/次,HRP标记的二抗(CST)常温孵育1 h,TBST洗涤3次,5 min/次,在暗室中将PVDF膜蛋白面浸入HRP-ECL发光液中,并使用ChemiDoc XRS成像系统显影。使用GAPDH作为内参。
1.6 Real-time PCR使用Trizol试剂提取组织和细胞中的总RNA。使用PrimeScriptTM RT Master Mix(Perfect Real-time)反转录试剂盒对所提取的RNA进行反转录。将反应物混匀后,放入MyCyclerTM普通PCR仪中进行反转录。当成功反转录为cDNA后,使用SYBR® Premix Ex TaqTM Ⅱ(TliRNaseH Plus)试剂盒进行Real-time quantitative PCR实验。其中引物序列为:Survivin,上游引物5'-CACCCCGGAGCGGATGG-3',下游引物5'-GTCATC TGGCTCCCAGCCTTC-3';AKT,上游引物5'-AGCG ACGTGGCTATTGTGAAG-3',下游引物5'-GCCATC ATTCTTGAGGAGGAAGT-3';PI3K,上游引物5'-TAT TTGGACTTTGCGACAAGACT-3',下游引物5'-TCG AACGTACTGGTCTGGATAG-3';GAPDH,上游引物5'-GGAGCGAGATCCCTCCAAAAT-3',下游引物5'-GAACCTGGAAGAGTCCGAAGTA-3'。其中GAPDH作为内参。
1.7 统计学方法使用Graphpad Prism 5.0版本软件分析两组之间的差异(two-tailed student's t检验),采用Pearson correlation分析Survivin与PI3K、AKT表达的相关性。当P<0.05认为差异有统计学意义。
2 结果 2.1 免疫组织化学和Western blot检测与正常皮肤相比,Survivin、PI3K和AKT蛋白在PV皮损组织KCs中表达上调(图 1)。
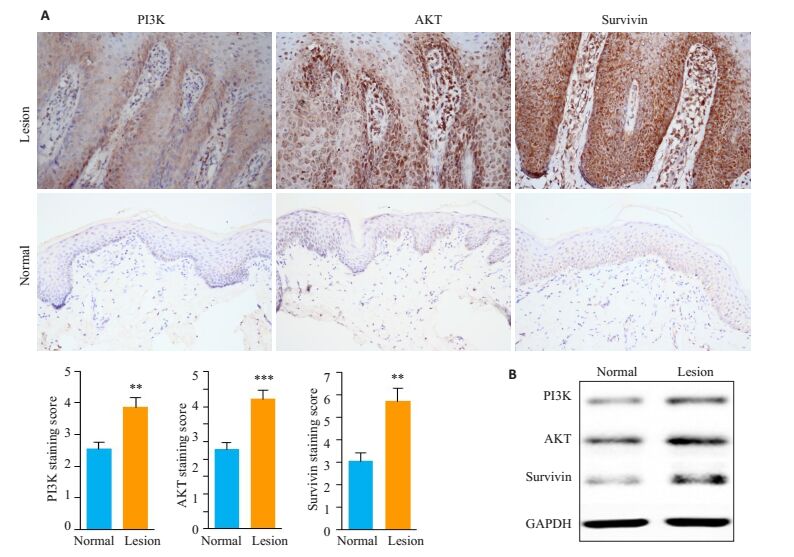
|
图 1 Survivin、PI3K和AKT在PV皮损KCs中的表达 Figure 1 Expression of survivin, PI3K and AKT in the keratinocytes inPV lesions. A: Immunohistochemical staining of survivin, PI3K and AKT in the keratinocytes in normal skin and PV lesions (Original magnification: × 400); B: Western blot analysis of survivin, PI3K and AKT in the keratinocytes in normal skin and PV lesions. **P < 0.01, ***P < 0.001 vs normal skin. |
Survivin和PI3K mRNA的表达呈正相关(r=0.4510;P=0.0351);Survivin和AKT mRNA表达呈正相关(r= 0.4423;P=0.0393,图 2)。
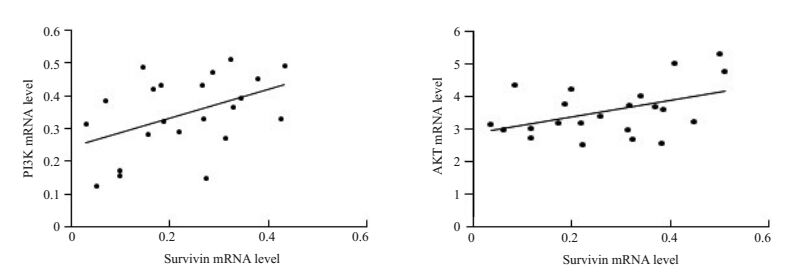
|
图 2 PV皮损KCs中Survivin、PI3K和AKT的相关性分析 Figure 2 Correlation analysis of survivin, PI3K and AKT in the keratinocytes in PV lesions. Left: Correlation analysis between survivin and PI3K mRNA; Right: Correlation analysis between survivin and AKT mRNA. |
在HaCaT细胞中,沉默AKT可以下调Survivin的表达(图 3)。
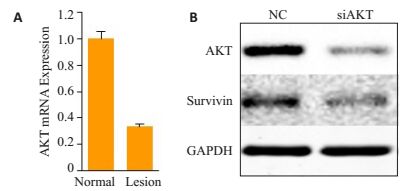
|
图 3 PV皮损KCs中PI3K/AKT信号通路和Survivin的相关性 Figure 3 Correlation between PI3K/AKT signaling pathway and survivin in the keratinocytes in PV lesions. A: Real-time quantitative PCR of AKT in HaCaT NC and AKT-deficient sublines; B: Western blot analysis of AKT and survivin in HaCaT NC and AKT deficient sublines. |
Survivin作为凋亡蛋白抑制因子(IAP)家族成员中抗凋亡作用最强的蛋白之一,目前广泛的在各种肿瘤组织中表达,如乳腺癌、口腔癌、肺癌和肝癌等[4-7]。大量研究发现Survivin在肿瘤中高表达且可以抑制肿瘤细胞的凋亡,从而促进肿瘤的发生、增殖和对放射治疗以及化学治疗的抵抗[6, 11-13]。PV是一种慢性炎症性、增殖性皮肤病,角质形成细胞增殖过度和凋亡的缺失不足是PV发生发展的机制之一[1-2]。目前有关Survivin在PV皮损中的表达水平尚有争议。Bowen等[14]发现最先报道Survivin在皮肤肿瘤和角质细胞增生皮损中表达增高,而在正常皮肤中表达较低。Simonetti等[15]报道Survivin和血管内皮生成因子(VEGF)在PV皮损中均表达上调。同样,Wang等[16]发现Survivin不仅在PV皮损中表达上调,且与抗菌肽β-defensin-3表达呈正相关。然而,Gunduz K等[17]报道PV皮损中survivin蛋白表达水平与正常皮肤中的表达水平没有差异。为了进一步明确survivin在PV皮损中的表达情况,我们在蛋白和mRNA水平上进行探究,发现survivin mRNA和蛋白水平在PV皮损中的表达水平均高于正常皮肤。
PI3K/AKT信号通路在细胞生长、增殖等功能中起重要的作用[18-19]。AKT是PI3K下游的靶蛋白,可以通过磷酸化其下游mTOR、Bad等蛋白,从而调控细胞的增殖和凋亡[20, 23]。大量研究证明,PI3K/AKT信号通路在肿瘤增殖和凋亡异常中起到重要作用[24-25]。在PV发生发展过程中,角质形成细胞的增殖和凋亡失衡起着重要的作用。Chamcheu等[9-10]发现在Imiquimod诱导的鼠PV样皮炎模型中,PI3K/AKT信号活性是增强的,进一步发现抑制PI3K/AKT信号通路可以缓解Imiquimod诱导的鼠PV样皮损。Jeon等[26]报道抑制PI3K/ AKT信号通路可以缓解皮肤的炎症反应。然而,PI3K和AKT在人PV皮损中的表达尚不清楚。在我们的研究中发现,与正常皮肤相比,PV皮损KCs中的PI3K和AKT的表达均上调,提示PI3K/AKT信号通路在PV发生发展中具有重要的作用。
PI3K/AKT信号通路参与了肿瘤细胞的增殖和凋亡异常。然而,在PV中,尚不清楚PI3K/AKT信号通路是否参与了促进角质形成细胞(KCs)增殖、抑制KCs凋亡的过程。本研究检测了PV皮损中Survivin、PI3K和AKTmRNA的表达,并对Survivin和PI3K以及Survivin和AKT进行了相关性分析。我们发现Survivin和PI3K的表达呈正相关;Survivin和AKT的表达也呈正相关,表明PI3K/AKT信号通路可能通过Survivin参与了PV皮损中KCs的凋亡受抑。另外,在体外细胞实验中,我们发现沉默HaCaT细胞AKT可以下调其Survivin蛋白的表达,提示KCs中AKT可以正向调控其Survivin的表达。
目前普遍认为,IL-23/Th17(白介素-23/辅助性T细胞17)轴的失调引起并加剧PV患者皮损中慢性炎症反应[27]。随着对银屑病发病机制研究的深入,人们发现各种细胞因子及炎性细胞、炎性介质在银屑病的发病中都不是单一发挥作用的,而是相互制约、相互影响,构成一个复杂的网络。在银屑病发病过程中,炎症、增殖与凋亡密切相关。
总之,我们的研究初步发现Survivin与PI3K、AKT在PV皮损KCs中蛋白表达均升高,并在mRNA表达水平呈正相关,沉默KCsAKT可以下调其Survivin蛋白的表达,表明PI3K/AKT信号通路可能通过凋亡抑制蛋白Survivin参与了PV的发生与发展,提示Survivin与PI3K/AKT信号通路可为银屑病治疗潜在的新靶点,可能为银屑病的治疗提供新的思路。
| [1] | Teraki Y, Shiohara T. Apoptosis and the skin[J]. Eur J Dermatol, 1999, 9(5): 413-25. |
| [2] | Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies[J]. J Allergy Clin Immunol, 2017, 140(3): 645-53. DOI: 10.1016/j.jaci.2017.07.004. |
| [3] | Boehncke WH, Brembilla NC. Unmet needs in the field of psoriasis: pathogenesis and treatment[J]. Clin Rev Allergy Immunol, 2017, [Epub ahead of print]. |
| [4] | Huang Q, Zeng Y, Lin H, et al. Transfection with Livin and SurvivinshRNA inhibits the growth and proliferation of non-small cell lung cancer cells[J]. Mol Med Rep, 2017, 16(5): 7086-91. |
| [5] | Li SX, Yang YQ, Ding YP, et al. Impacts of survivin and caspase-3 on apoptosis and angiogenesis in oral cancer[J]. Oncol Lett, 2017, 14(3, B): 3774-9. |
| [6] | Taglieri L, De Iuliis F, Giuffrida AA, et al. Resistance to the mTOR inhibitor everolimus is reversed by the downregulation of survivin in breast cancer cells[J]. Oncol Lett, 2017, 14(3, B): 3832-8. |
| [7] | Silke J, Vince J. IAPs and cell death[J]. Curr Top Microbiol Immunol, 2017, 403: 95-117. |
| [8] | Guerrero-Zotano A, Mayer IA, Arteaga CL. PI3K/AKT/mTOR: role in breast cancer progression, drug resistance, and treatment[J]. Cancer Metastasis Rev, 2016, 35(4): 515-24. DOI: 10.1007/s10555-016-9637-x. |
| [9] | Chamcheu JC, Chaves-Rodriquez MI, Adhami VM, et al. Upregulation of PI3K/AKT/mTOR, FABP5 and PPAR beta/delta in Human Psoriasis and Imiquimod-induced Murine Psoriasiform Dermatitis Model[J]. Acta DermVenereol, 2016, 96(6): 854-6. |
| [10] | Chamcheu JC, Adhami VM, Esnault S, et al. Dual inhibition of PI3K/Akt and mTOR by the dietary antioxidant, delphinidin, ameliorates psoriatic features in vitro and in an Imiquimod-Induced Psoriasis-Like disease in mice[J]. Antioxid Redox Signal, 2017, 26(2): 49-69. DOI: 10.1089/ars.2016.6769. |
| [11] | Lee EY, Gong EY, Shin JS, et al. Human breast cancer cells display different sensitivities to ABT-263 based on the level of survivin[J]. Toxicol in vitro, 2017, 46: 229-36. |
| [12] | Gu F, Li L, Yuan QF, et al. Down-regulation of survivin enhances paclitaxel-induced Hela cell apoptosis[J]. Eur Rev Med Pharmacol Sci, 2017, 21(15): 3504-9. |
| [13] | Zhang ZX, Wang TY, Liu ZQ, et al. Small interfering RNA target ing of the survivin gene inhibits human tumor cell growth in vitro[J]. ExpTher Med, 2017, 14(1, A): 35-42. |
| [14] | Bowen AR, Hanks AN, Murphy KJ, et al. Proliferation, apoptosis, and survivin expression in keratinocytic neoplasms and Hyperplasias[J]. Am J Dermatopathol, 2004, 26(3): 177-81. DOI: 10.1097/00000372-200406000-00001. |
| [15] | Simonetti O, Lucarini G, Campanati AA, et al. VEGF, survivin and NOS overexpression in psoriatic skin: Critical role of nitric oxide synthases[J]. J Dermatol Sci, 2009, 54(3): 205-8. DOI: 10.1016/j.jdermsci.2008.12.012. |
| [16] | Wang F, Zhang X, Xia P, et al. Enhancement of mRNA expression of survivin and human beta-defensin-3 in lesions of psoriasis vulgaris[J]. Eur J Dermatol, 2016, 26(1): 28-33. |
| [17] | Gunduz K, Temiz P, Gencoglan G, et al. Expression of nuclear factor kappa B and survivin in psoriasis[J]. ISRN Dermatol, 2012, 2012: 257059. |
| [18] | Keppler-Noreuil KM, Parker VE, Darling TN, et al. Somatic overgrowth disorders of the PI3K/AKT/mTOR pathway & therapeutic strategies[J]. Am J Med Genet C Semin Med Genet, 2016, 172(4): 402-21. DOI: 10.1002/ajmg.c.31531. |
| [19] | Yu JS, Cui W. Proliferation, survival and metabolism: the role of PI3K/AKT/mTORsignalling in pluripotency and cell fate determination[J]. Development, 2016, 143(17): 3050-60. DOI: 10.1242/dev.137075. |
| [20] | Hu BW, Lv X, Gao F, et al. Downregulation of DEPTOR inhibits the proliferation, migration, and survival of osteosarcoma through PI3K/Akt/mTOR pathway[J]. Onco Targets Ther, 2017, 10: 4379-91. DOI: 10.2147/OTT. |
| [21] | Wang ZY, Zhou LQ, Zheng XT, et al. Autophagy protects against PI3K/Akt/mTOR-mediated apoptosis of spinal cord neurons after mechanical injury[J]. Neurosci Lett, 2017, 656: 158-64. DOI: 10.1016/j.neulet.2017.07.036. |
| [22] | Feng XN, Jiang JJ, Shi SH, et al. Knockdown of miR-25 increases the sensitivity of liver cancer stem cells to TRAIL-induced apoptosis via PTEN/PI3K/Akt/Bad signaling pathway[J]. Int J Oncol, 2016, 49(6): 2600-10. DOI: 10.3892/ijo.2016.3751. |
| [23] | Ma YF, Qin HD, Cui YF. MiR-34a targets GAS1 to promote cell proliferation and inhibit apoptosis in papillary thyroid carcinoma via PI3K/Akt/Bad pathway[J]. BiochemBiophys Res Commun, 2013, 441(4): 958-63. DOI: 10.1016/j.bbrc.2013.11.010. |
| [24] | Wang YY, Zhao M, Liu JY, et al. miRNA-125b regulates apoptosis of human non-small cell lung cancer via the PI3K/Akt/GSK3 beta signaling pathway[J]. Oncol Rep, 2017, 38(3): 1715-23. |
| [25] | Lin YT, Wang HC, Hsu YC, et al. Capsaicin induces autophagy and apoptosis in human nasopharyngeal carcinoma cells by downregulating the PI3K/AKT/mTOR pathway[J]. Int J MolSci, 2017, 18(7): E1343. |
| [26] | Jeon YJ, Kim BH, Kim S, et al. Rhododendrin ameliorates skin inflammation through inhibition of NF-kappa B, MAPK, and PI3K/ Akt signaling[J]. Eur J Pharmacol, 2013, 714(1/3): 7-14. |
| [27] | Song HS, Kim SJ, Park TI, et al. Immunohistochemical comparison of IL-36 and the IL-23/Th17 axis of generalized pustular psoriasis and acute generalized exanthematous pustulosis[J]. Ann Dermatol, 2016, 28(4): 451-6. DOI: 10.5021/ad.2016.28.4.451. |
 2017, Vol. 37
2017, Vol. 37

