2. 上海交通大学医学院附属新华医院, 上海 200092;
3. 顺德职业技术学院医药卫生学院, 广东 佛山 528000;
4. 广州市刑事科学技术研究所//广东省法医遗传学重点实验室, 广东广州 510030
2. Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine, Shanghai 200092, China;
3. Guangzhou Forensic Science Institute, Guangzhou 510030, China;
4. ${affiliationVo.addressStrEn}
甲基苯丙胺(METH)属于苯丙胺类神经兴奋剂,在世界范围内被广泛滥用,是当前对公共卫生和社会危害最大的毒品之一[1-2]。研究表明METH主要作用于中枢神经系统,结构与儿茶酚胺类神经递质相似,主要表现为多巴胺等单胺类神经末梢的损伤,可出现与帕金森病等神经退行性疾病相似的病理改变[3-4]。近年来研究证实,多种机制参与了METH的神经毒性,主要包括氧化应激[5]、神经元凋亡与自噬[6-7]、兴奋性毒性[8]、线粒体功能障碍[9]等,其中氧化应激是METH所致神经毒性损伤的重要作用机制之一[10]。二硫键异构酶(PDI)是一种内质网滞留蛋白,在稳定蛋白质的三维结构中起着重要作用,同时还具有分子伴侣活性,能抑制错误折叠蛋白的聚集[11]。在神经退行性疾病的研究中发现,PDI可通过抑制错误折叠蛋白的聚集而起到保护神经元的作用。但PDI易与氧化应激产生的过量的NO结合,形成S亚硝基化PDI(PDI-SNO),导致其构象改变和功能障碍[12]。α-突触核蛋白(α-SN)既参与正常突触功能的维持,又与帕金森病等神经退行性疾病有关,在生理状态下处于无序伸展状态,没有细胞毒性,而在病理状态下,可引起α-SN错误折叠,在细胞内聚集形成纤维化或寡聚体[13-14]。研究认为聚集状态的α-SN能促进细胞的氧化应激增强,对神经元产生毒性作用,同时氧化应激又促进α-SN发生聚集,形成恶性循环[15]。前期体内外研究发现,METH可以导致NO水平明显升高,氧化应激显著增强[16-17]。PC12细胞在METH处理后,PDI发生S亚硝基化,α-SN表达明显升高并产生神经毒性[18],而关于METH对小鼠大脑的神经退行性病变的作用及其机制,尤其是PDI亚硝基化对METH致小鼠相关脑区α-SN表达的影响,未见相关报道。
基于此,本研究拟建立METH亚急性中毒小鼠模型,METH与一氧化氮合酶(NOS)抑制剂N-硝基-L-精氨酸(L-NNA)共同处理小鼠,检测NOS、NO、PDI、PDISNO和α-SN等指标,研究在METH致神经毒性中PDISNO对异常表达的α-SN的影响,进一步完善METH诱导的α-SN聚集的毒性机制,为治疗METH引起的神经毒性损伤提供理论基础。
1 材料和方法 1.1 材料和试剂C57 BL/6小鼠购自广东省医学实验动物中心,盐酸甲基苯丙胺购自中国药品生物制品鉴定所,N-硝基-L-精氨酸(L-NNA)购自Sigma,兔抗鼠α-SN多克隆抗体购自CST,Cat no.#2642,兔抗鼠PDI多克隆抗体购自CST,Cat no. #2446,羊抗兔IgG-HRP购自北京锐抗公司,NO检测试剂盒购自南京建成生物工程研究所,其他试剂均为国产分析纯。
1.2 实验方法 1.2.1 METH亚急性中毒小鼠模型及药物处理取6周龄雄性C57小鼠40只,分笼饲养于22 ℃恒温动物房内,自由进水进食,建立12 h黑夜和白昼的交替循环,让小鼠适应环境1周后随机分成4组(n=10只/组),对照(CON)组、L-NNA组、METH组和METH+L-NNA组。L-NNA组和METH组给予单次腹腔注射L-NNA(8 mg/kg)或METH(15 mg/kg),间隔12 h注射1次,共8次。METH+L-NNA组腹腔注射L-NNA 8 mg/kg,0.5 h后给予METH 15 mg/kg。对照组小鼠同等条件下腹腔注射生理盐水300 μL/只。最后1次给药24 h后麻醉、断颈、分离纹状体和海马脑区。
1.2.2 指标检测Western Blotting法检测各组内NOS、PDI、α-SN等指标,生物素转化法对各组PDI-SNO进行转化后进行Western Blotting。NO含量用NO检验试剂盒检测。
1.3 统计方法采用GraphPad Prism software Version 5.0a(GraphPad Software Inc., 美国)软件进行统计学分析,数据以均数±标准差表示,组间比较采用单因素方差分析,P < 0.05为差异有统计学意义。
2 结果 2.1 L-NNA对METH所致的小鼠脑区NOS表达影响Western blotting检测纹状体和海马脑区内NOS表达情况,结果显示,METH组NOS表达较CON组显著增高(P < 0.05),且纹状体L-NNA组NOS表达较CON组明显降低(P < 0.05);而当L-NNA与METH共同处理C57小鼠时,L-NNA可显著降低METH诱导的NOS的上升(P < 0.05,图 1)。
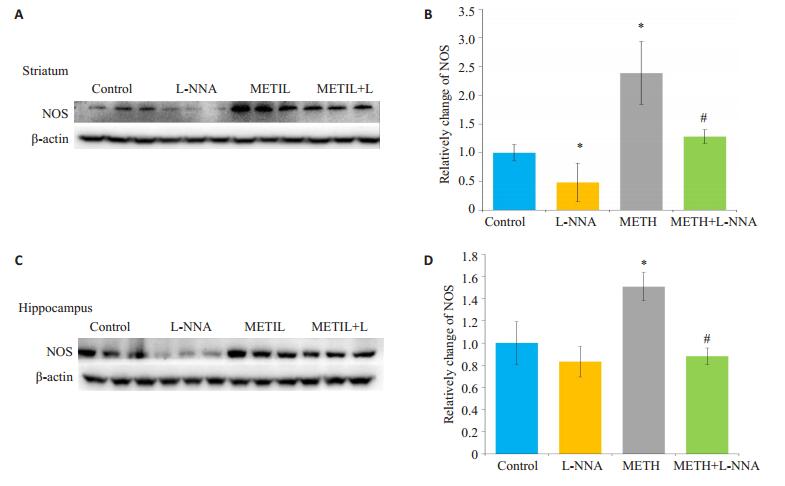
|
图 1 小鼠脑区各组NOS表达变化 Figure 1 Changes of NOS protein expression in the mouse brain in different groups. A, C: Western blotting for detecting NOS in the striatum and hippocampus, respectively; B, D: Gray values of NOS determined by Image J software. Data represent average from 3 independent tests. *P < 0.05 vs control group; #P < 0.05 vs METH group. |
NO检验试剂盒检测纹状体和海马脑区NO含量,结果表明,METH组NO含量较CON组显著增多(P < 0.05);L-NNA组NO含量与CON组相比无明显差异(P > 0.05);当L-NNA与METH共同处理C57小鼠时,L-NNA可显著降低METH诱导的NO含量的上升(P < 0.05,图 2)。
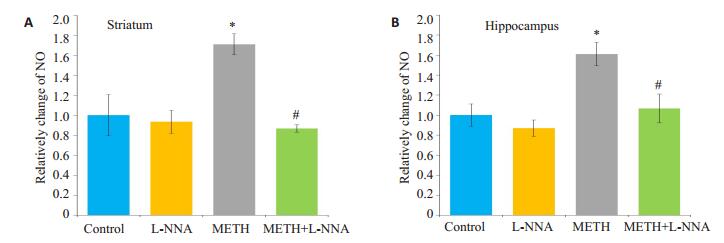
|
图 2 小鼠脑区各组NO含量变化 Figure 2 Changes of NO level in the striatum (A) and hippocampus (B) of the mice in different groups. *P < 0.05 vs control group; #P < 0.05 vs METH group. |
采用生物素转化法将纹状体和海马脑区内PDISNO标记并转化,再利用Western blotting检测转化后PDI蛋白表达变化。结果表明,两脑区内METH组与CON组相比,可使PDI亚硝基化表达显著升高(P < 0.05);L-NNA与METH共同处理组PDI-SNO含量较METH组明显降低(P < 0.05,图 3)。
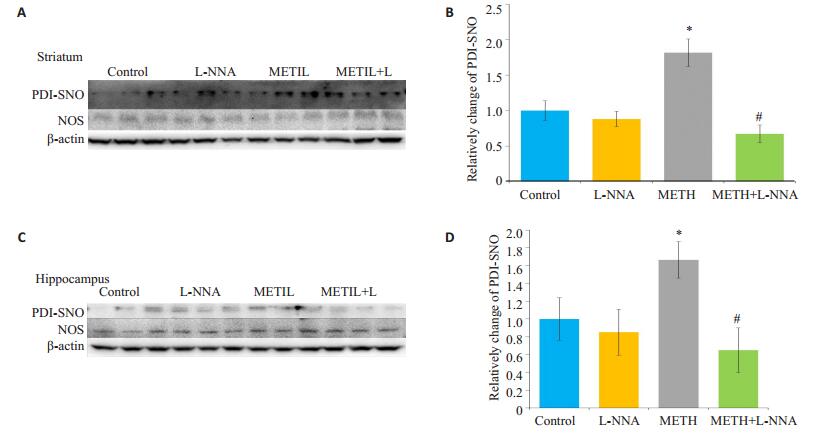
|
图 3 小鼠脑区各组PDI-SNO表达变化 Figure 3 Changes of PDI-SNO level in the mouse brain in different groups. A, C: Western blotting for detecting PDISNO in the striatum and hippocampus regions, respectively; B, D: Gray values of PDI-SNO in the striatum and hippocampus determined by Image J software analysis, respectively. Data represent average from 3 independent tests. *P < 0.05 vs control group; #P < 0.05 vs METH group. |
Western blotting检测纹状体和海马脑区α-SN表达情况。结果表明,METH组α-SN含量均比CON组显著升高(P < 0.05),与前期的体外实验研究结果相一致;L-NNA组内α-SN含量与CON组无显著差异(P > 0.05),L-NNA与METH共处理C57小鼠,可有效抑制METH导致的α-SN的升高(P < 0.05,图 4)。
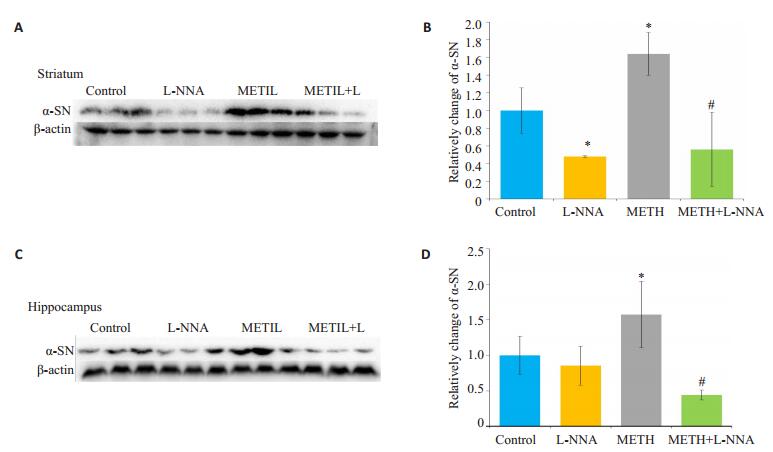
|
图 4 小鼠脑区各组α-SN表达变化 Figure 4 Changes of α-SN level in the mouse brain in different groups. A, C: Western blotting for detecting α-SN in the striatum and hippocampus, respectively; B, D: Analysis of gray values of α-SN using Image J software. Data represent average from 3 independent tests. *P < 0.05 vs control group; #P < 0.05 vs METH group. |
METH作为世界上滥用最广泛的苯丙胺类兴奋剂,具有药物依赖性、中枢兴奋性、致幻、食欲抑制等药理、毒理学特性[19]。而由METH造成的神经退行性样改变[20-22]一直以来是我们关注的热点问题。
研究表明,黑质区多巴胺能神经元的丧失与蛋白聚集体——路易小体的形成是PD的两大病理特征,α-SN是Lewy体的重要组成成分,错误折叠的α-SN的聚集被认为是帕金森病(PD)发病机制的关键环节[23]。在METH致神经退行性样改变的研究过程中,METH作用后α-SN在纹状体、海马等脑区表达异常升高,并已证实α-SN在METH所致的氧化应激、多巴胺代谢障碍和线粒体功能丧失等神经毒性机制中起到关键作用。本研究前期使用METH处理分化的大鼠嗜铬细胞瘤PC12细胞,结果发现PDI发生显著的S亚硝基化,L-NNA与METH共同处理细胞,可通过抑制NOS,减少细胞内NO的生成量,进而显著降低METH所致的PDI-SNO程度,缓解METH所致的α-SN的升高,从而改善METH所致神经毒性作用[18, 24-25]。尽管我们前期研究发现METH所致的PDI-SNO对α-SN异常聚集有影响,但由于实验以PC12细胞系为模型,并不能真实反映METH作用后大脑神经元的毒性损伤变化,所以,为进一步阐明PDI-SNO在METH作用后对中枢神经系统内α-SN异常表达的影响,在体外研究基础上,本次实验利用小鼠METH亚急性中毒模型对神经毒性损伤明显的纹状体、海马脑区进行相关研究。并首次将NOS抑制剂LNNA应用于体内实验。结果发现,给予METH处理,在海马、纹状体区两个脑区,NO含量与PDI-SNO明显增加同时伴随α-SN表达升高;使用NOS抑制剂L-NNA与METH共处理C57小鼠,NO含量、PDI-SNO和α-SN表达均显著降低这表明L-NNA抑制NOS表达后,降低NO浓度从而减轻METH所致的神经毒性。这也间接证明了METH作用后NO含量升高,致使相关脑区氧化应激损伤[26],PDI发生显著S亚硝基化,导致α-SN表达升高[27-29]。上述动物相关脑区的实验结果对我们前期的研究发现更有说服力。
结合我们的体内外研究,可以明确PDI是降解异常聚集α-SN的重要蛋白。METH作用后NO含量升高,引起氧化应激损伤,导致PDI发生S亚硝基化,使其功能丧失,错误折叠的α-SN降解障碍而发生异常聚集,最终产生神经毒性。此外,我们认为关键功能蛋白的硝基化也是METH神经毒性损伤作用的重要机制之一,以往研究报道METH可通过DDAH1/ADMA/NOS通路引起蛋白质硝基化水平升高导致神经毒性[30-31],关键蛋白如谷胱甘肽S-转移酶P1的硝基化可激活细胞周期素依赖蛋白激酶5导致氧化应激损伤,加剧细胞的死亡[23]。由此,我们下一步将深入研究METH作用后氧化应激是否可以直接导致α-SN发生硝基化而影响其生物学功能,为阐明METH神经退行性病变的毒性机制奠定良好的基础。
| [1] | 郝柳, 罗涛, 唐爱国, 等. 甲基苯丙胺滥用的研究进展[J]. 中国药物滥用防治杂志, 2015, 21(5): 302-6. |
| [2] | Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death[J]. Brain Res Rev, 2009, 60(2): 379-407. DOI: 10.1016/j.brainresrev.2009.03.002. |
| [3] | United Nations Office on Drugs and Crime. World Drug Report[R]. New York, NY:United Nations Publications:United Nations Office on Drugs and Crime.2016. |
| [4] | Carvalho M, Carmo H, Costa VM, et al. Toxicity of amphetamines:an update[J]. Arch Toxicol, 2012, 86(8): 1167-231. DOI: 10.1007/s00204-012-0815-5. |
| [5] | Hozumi H, Asanuma M, Miyazaki I, et al. Protective effects of interferon-gamma against methamphetamine-induced neurotoxicity[J]. Toxicol Lett, 2008, 106(1): 175P. |
| [6] | Chen L, Huang EP, Wang HJ, et al. RNA interference targeting alpha-synuclein attenuates methamphetamine-induced neurotoxicity in SH-SY5Y cells[J]. Brain Res, 2013, 1521(8): 59-67. |
| [7] | Li B, Chen R, Chen L, et al. Effects of DDIT4 in MethamphetamineInduced autophagy and apoptosis in dopaminergic neurons[J]. Mol Neurobiol, 2017, 54(3): 1642-60. DOI: 10.1007/s12035-015-9637-9. |
| [8] | Tats DA, Yamamoto BK. Chronic stress enhances metham. phetamine-induced extracellalar glutamate and exeitotoxicityinthe rat striatum[J]. Synapse, 2008(2): 325-36. |
| [9] | Chen CX, Qincao L, Xu JT, et al. Role of PUMA in methamphetamine-induced neuronal apoptosis[J]. Toxicol Lett, 2016, 240(1): 149-60. DOI: 10.1016/j.toxlet.2015.10.020. |
| [10] | Zhang X, Dong F, Mayer GE, et al. Selective inhibition of cyclooxygenase-2 exacerbates methamphetamine-induced dopamine depletion in the striatum in rats[J]. Neuroscience, 2007, 150(4): 950-8. DOI: 10.1016/j.neuroscience.2007.09.059. |
| [11] | Lu J, Holmgren A. The thioredoxin superfamily in oxidative protein folding[J]. Antioxid Redox Signal, 2014, 21(3): 457-70. DOI: 10.1089/ars.2014.5849. |
| [12] | Nakamura T, Lipton SA. Protein S-Nitrosylation as a therapeutic target for neurodegenerative diseases[J]. Trends Pharmacol Sci, 2016, 37(1): 73-84. DOI: 10.1016/j.tips.2015.10.002. |
| [13] | Lee HJ, Lee SJ. Characterization of cytoplasmic alpha-synuclein aggregates. Fibril formation is tightly linked to the inclusionforming process in cells[J]. J Biol Chem, 2002, 277(50): 48976-83. DOI: 10.1074/jbc.M208192200. |
| [14] | Kalia LV, Kalia SK, Mclean PJ, et al. alpha-Synuclein oligomers and clinical implications for parkinson disease[J]. Ann Neurol, 2013, 73(2): 155-69. DOI: 10.1002/ana.23746. |
| [15] | 沈原, 赵永波, 刘功禄, 等. α-突触核蛋白的表达与氧化应激水平的相互影响[J]. 中国神经免疫学和神经病学杂志, 2011, 18(3): 174-7. |
| [16] | 徐静涛, 张付, 杨幸怡, 等. 甲基苯丙胺所致的大鼠神经毒性损伤及nNOS抑制剂的保护作用[J]. 中国药理学通报, 2014, 30(8): 1101-6. |
| [17] | Cadet JL. Roles of glutamate, nitric oxide, oxidative stress, and apoptosis in the neurotoxicity of methamphetamine[M]. Humana Press, 2002: 201-10. |
| [18] | Wu XF, Wang AF, Chen L, et al. S-nitrosylating protein disulphide isomerase mediates alpha-synuclein aggregation caused by methamphetamine exposure in PC12 cells[J]. Toxicol Lett, 2014, 230(1): 19-27. DOI: 10.1016/j.toxlet.2014.07.026. |
| [19] | Yu SB, Zhu L, Shen Q, et al. Recent advances in methamphetamine neurotoxicity mechanisms and its molecular pathophysiology[J]. Behav Neurol, 2015, 12(3): 1-11. |
| [20] | Volkow ND, Chang L, Wang GJ, et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers[J]. Am J Psychiatry, 2001, 158(3): 377-82. DOI: 10.1176/appi.ajp.158.3.377. |
| [21] | Callaghan RC, Cunningham JK, Sykes JA. Increased risk of Parkinson's disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs[J]. Drug Alcohol Depend, 2012, 120(1/3): 35-40. |
| [22] | Imam SZ, Ali SF. Aging increases the susceptiblity to methamphetamine-induced dopaminergic neurotoxicity in rats:correlation with peroxynitrite production and hyperthermia[J]. J Neurochem, 2001, 78(5): 952-9. DOI: 10.1046/j.1471-4159.2001.00477.x. |
| [23] | Cavaliere F, Cerf L, Dehay B, et al. In vitro ɑ-synuclein neurotoxicity and spreading among neurons and astrocytes using Lewy body extracts from Parkinson disease brains[J]. Neurobiol Dis, 2017, 103(7): 101-12. |
| [24] | Li Y, Chung KK. Involvement of S-Nitrosylation in neurodegeneration[J]. Protein Reviews, 2011, 13(4): 79-95. |
| [25] | Wen D, An ML, Gou HY, et al. Cholecystokinin-8 inhibits methamphetamine-induced neurotoxicity via an anti-oxidative stress pathway[J]. Neurotoxicology, 2016, 57(8): 31-8. |
| [26] | Friend DM, Son JH, Keefe KA. Expression and activity of nitric oxide synthase isoforms in Methamphetamine-Induced striatal dopamine toxicity[J]. J Pharmacol Exp Ther, 2013, 344(2): 511-21. DOI: 10.1124/jpet.112.199745. |
| [27] | 陈汉, 王慧君, 李学锋, 等. 甲基苯丙胺对大鼠脑组织中NO, SOD和MDA的影响[J]. 中国药物依赖性杂志, 2007, 16(2): 102-4. |
| [28] | 吴小芳, 王爱枫, 邱平明. 甲基苯丙胺对PC12细胞中二硫键异构酶S亚硝基化的影响[J]. 南方医科大学学报, 2017, 37(1): 93-6. |
| [29] | Li XE, Wang HJ, Qiu PM, et al. Proteomic profiling of proteins associated with methamphetamine-induced neurotoxicity in different regions of rat brain[J]. Neurochem Int, 2008, 52(1/2): 256-64. |
| [30] | Zhang F, Chen L, Liu C, et al. Up-regulation of protein tyrosine nitration in methamphetamine-induced neurotoxicity through DDAH/ADMA/NOS pathway[J]. Neurochem Int, 2013, 62(8): 1055-64. DOI: 10.1016/j.neuint.2013.03.016. |
| [31] | Song JW, Liu ZJ, Tan XH, et al. Involvement of DDAH/NOS pathway in methamphetamine-induced neurotoxicity in rat striatum[J]. 华西药学杂志, 2010, 25(6): 679-81. |
 2017, Vol. 37
2017, Vol. 37

