2. 中国科学院深圳先进技术研究院保罗. C. 劳特伯生物医学成像中心, 广东 深圳 518055;
3. 南方医科大学附属广东省第二人民医院超声医学科, 广东 广州 510317
2. Paul C. Lauterbur Research Center for Biomedical Imaging, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, China;
3. Department of Ultrasonography, Guangdong Second Provincial General Hospital Affiliated to Southern Medical University, Guangzhou, 510317, China
类风湿关节炎(RA)是一种以关节滑膜慢性炎症为特点,具有高度致残性的全身性自身免疫性疾病[1-2]。其基本病理改变是关节滑膜增生伴新生血管形成及关节的炎症,终而导致关节的破坏、畸形和功能丧失,严重影响人们的生活质量[3-4],虽然目前的抗风湿药在一定程度上能改善RA病情,但它们到达病变关节的靶向性差、浓度低,全身用药的副作用明显且疗程长[5-7]。因此,提高RA病变关节的药物浓度,增强药物治疗效果,同时降低全身副作用,是当前RA治疗中亟待解决的难题之一。
近年来,许多研究将普通纳米载药体系用于RA的诊疗过程中,取得了一定的疗效,但在治疗过程中存在药物无法主动控释,药物聚集程度无法监测等问题[8-9]。声学脂质体(ELIP)作为一种多功能的纳米载药体,具有载药量高、声学响应性好等特性,除了可以靶向传递药物外,还能在超声的作用下定点控释药物,有助于实现病变组织药物控释和渗透,进一步增强药物的局部治疗效果,其在血栓、肿瘤等疾病治疗中都取得了良好的效果[10-13]。因此,本研究以RA发病及病程发展密切相关的αvβ3受体为靶点,设计与构建集治疗与成像为一体的载甲氨喋呤(MTX)和吲哚菁绿(ICG)声学脂质体(iRGD-MTX-ICG-ELIP)这一多功能诊疗颗粒,将其用于治疗RA,初步观察其靶向性及其在病变关节的聚集,检测其联合低频超声体外抑制MH7A的增殖效果,为进一步开展荧光成像引导下超声定点控释药物、靶向治疗RA实验研究奠定基础。这一设想目前尚未见文献报道。
1 材料和方法 1.1 主要试剂与仪器二棕榈酰磷脂酰胆碱(DPPC, Avanti Polar Lipids),胆固醇(Chol, Sigma-Aldrich, USA),聚乙二醇2000修饰的二硬脂酰基磷脂酰乙醇胺(DSPEPEG2000, Avanti Polar Lipids),iRGD多肽(吉尔生化上海有限公司),磷酸盐缓冲液(PBS),甲氨蝶呤(MTX,Aladdin),吲哚菁绿(ICG,上海克拉玛尔公司);人脐静脉内皮细胞系(HUVECs,美国模式培养物保藏所),滑膜细胞(MH7A,日本生物研究所),SPF级DBA/1J雌性小鼠(广东省动物中心,动物合格证号11400700103055,使用单位许可证号SYXK粤2012-0119);水浴超声振荡器(中国深圳市洁泰超声洗净设备有限公司),台式离心机(德国Sigma),紫外分光光度计(美国PerkinElmer),马尔文激光测径仪(英国Malvern),激光共聚焦显微镜(德国Leica),任意波形信号发生器(美国Tektronix);1.0 MHz超声探头(美国ValpeyFisher);酶标仪(美国BioTek)。
1.2 iRGD-MTX-ICG-ELIP的制备iRGD多肽和DSPE-PEG2000-Mal按1.5:1质量溶于超纯水中,冰浴环境反应24 h后,透析48 h滤除杂质,再将反应液置于-80 ℃冰箱冷冻,冷冻后再冷冻干燥48 h即得DSPE-PEG2000-iRGD。采用薄膜水化法和冷冻冻干法制备iRGD-MTX-ICG-ELIP:称取DPPC 30 mg、Chol 20 mg、DSPE-PEG2000 18 mg和DSPEPEG2000-iRGD 10 mg溶于适量三氯甲烷,37 ℃减压旋转蒸发成均匀的透明薄膜;称取MTX和ICG各1.5 mg溶于适量PBS,所得的溶液用以水化薄膜;加入0.32 mol/L的甘露醇,水浴超声振荡至透明后将其置于-80 ℃冰箱,之后冷冻干燥48 h,得iRGD-MTX-ICG-ELIP。同样的方法制备非靶向载MTX和ICG的声学脂质体(MTX-ICG-ELIP)。
1.3 iRGD-MTX-ICG-ELIP表征检测透射电镜(TEM)观察磷钨酸负染法处理后的iRGD-MTX-ICG-ELIP形态,Malvern激光测径仪检测其大小、分布及表面电荷等特性。
1.4 药物包封率检测分别配置MTX、ICG标准溶液,用紫外分光光度计测定它们的波长并绘制标准曲线,根据标准曲线计算超滤后iRGD-MTX-ICG-ELIP和MTX-ICG-ELIP中MTX和ICG的包封率,包封率(%)=(C总-C游)/C总×100%,C总为加入iRGD-MTX-ICG-ELIP中的药物量,C游为通过超滤法去除的游离药物量。
1.5 超声控释药物实验取iRGD-MTX-ICG-ELIP混悬液分为14组,每组3份(1 mL/份),2组为非超声组,超声组条件为1 MHz的低频聚焦探头,连续波,功率放大100%。选取6组固定超声作用时间120 s,设置0.3、0.4、0.5、0.6、0.7、0.9 MPa 6种不同超声强度;另6组则固定超声强度为0.5 MPa,设置60、90、120、150、180、240 s超声作用时间进行处理。非超声组中的一组超速离心后取上清,用紫外分光光度计测上清中MTX和ICG的吸光度并计算其浓度(Ci),用同样的方法计算超声处理组各组间的药物浓度(Cus),另一非超声组则用80%的甲醇全部裂解,测裂解后的药物浓度(Cf)。药物释放率=(Cus-Ci)/(Cf-Ci)×100%。
1.6 细胞摄取实验取对数生长期的HUVECs细胞2×104/孔的贴壁生长于6孔板中培养24 h,加入同浓度的iRGD-MEXICG-ELIP、MTX-ICG-ELIP、PBS作空白对照,37 ℃温箱孵育6 h后用DAPI染液染细胞核,于激光共聚焦显微镜下观察各组与HUVECs的结合效率;同样将1×105/孔HUVECs贴壁生长于6孔板中培养24 h,加入同浓度iRGD-MTX-ICG-ELIP、MTX-ICG-ELIP,游离ICG药物,PBS作空白对照,孵育6 h后,细胞离心重悬,流式细胞仪测定各组摄取情况。
1.7 RA小鼠模型的构建及活体成像实验选体质量18~22 g的雌性DBA/1小鼠,采用牛Ⅱ型胶原混合佐剂法诱导的RA模型。造模成功后,将RA小鼠随机分为2组,每组5只,按0.2 mg/kg剂量于尾静脉注射MTX-ICG-ELIP和iRGD-MTX-ICG-ELIP混悬液2 h后,置于近红外活体成像下观察。
1.8 体外抑制MH7A增殖实验将MH7A按1×104/孔的浓度接种于96孔板生长贴壁后,设置对照组、单纯超声组、iRGD-MTX-ICG-ELIP组,iRGD-MTX-ICG-ELIP联合超声组,每组5个复孔(加入的iRGD-MTX-ICG-ELIP浓度为40 μg/mL,需超声的组别选用1 MHz聚焦探头,连续波,超声辐照2 min,超声作用强度0.5 MPa),孵育3 h后向板孔里面加入IL-1β(5 ng/mL)进行诱导,细胞再继续培养3 h,向每孔加入10 μL的CCK-8试剂,2 h后在450 nm的吸收波长下用酶标仪定量检测各组间MH7A增殖情况。
1.9 统计学分析实验数据采用SPSS 13.0统计软件进行统计学分析,实验结果以均数±标准差表示,组间差异采用单因素方差分析,P < 0.05判定为差异有统计学意义。
2 结果 2.1 iRGD-MTX-ICG-ELIP表征本研究制备的iRGD-MTX-ICG-ELIP为浅绿色粉末,溶于PBS后为均匀乳液;TEM观察到的iRGDMTX-ICG-ELIP为类圆形(图 1B)。Malvern激光测径仪测得iRGD-MTX-ICG-ELIP和MTX-ICG-ELIP的平均粒径分别为134.4±17.61 nm和116.36±15.86 nm,Zeta电位均在-10 mV左右(表 1)。
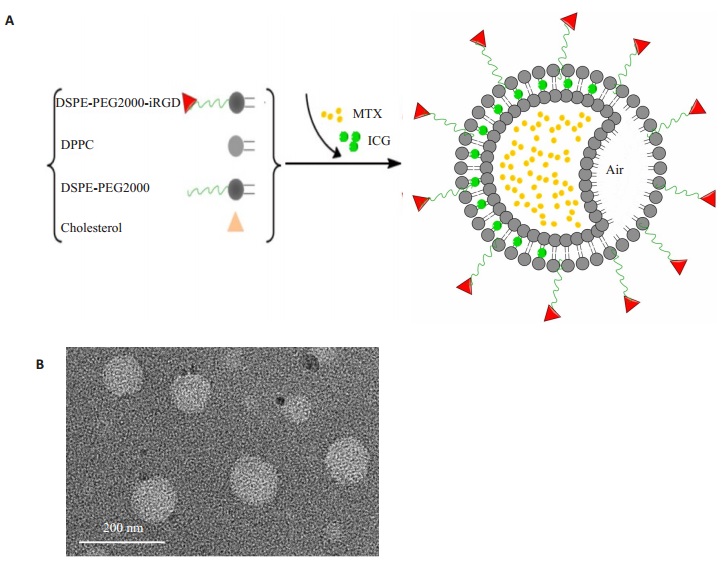
|
图 1 iRGD-MTX-ICG-ELIP表征 Figure 1 Characterization of iRGD-MTX-ICGELIP. A: Schematic diagram of the synthesis of iRGD-MTX-ICG-ELIP; B: TEM image of iRGD-MTX-ICG-ELIP; C: Size distribution of iRGD-MTX-ICG-ELIP by dynamic light scattering. |
| 表 1 iRGD-MTX-ICG-ELIP和MTX-ICG-ELIP的一般特性 Table 1 General properties of iRGD-MTX-ICG-ELIP and MTX-ICG-ELIP |
根据紫外分光光度计检测,MTX的最大吸收波长在303 nm,ICG的最大吸收波长在780 nm。以浓度为横坐标,吸光度为纵坐标,得线性回归方程:MTX为y=0.059x -0.02,R2=0.997;ICG为y=0.047x + 0.05,R2=0.990。iRGD-MTX-ICG-ELIP中MTX和ICG的平均包封率均大于60%(表 1)。
2.3 iRGD-MTX-ICG-ELIP联合超声控释药物结果体外低频超声控释药发现,iRGD-MTX-ICG-ELIP中MTX和ICG的释放量有随超声作用强度增强和作用时间延长而增多的趋势,而且当固定超声作用时间为120 s,超声作用强度达0.9 MHz时,MTX的释放率可达到85%,ICG的释放率达75%;而固定超声作用强度为0.5 MPa,作用时间240 s时,MTX释放率达58%,ICG的释放率可达73%(图 2)。
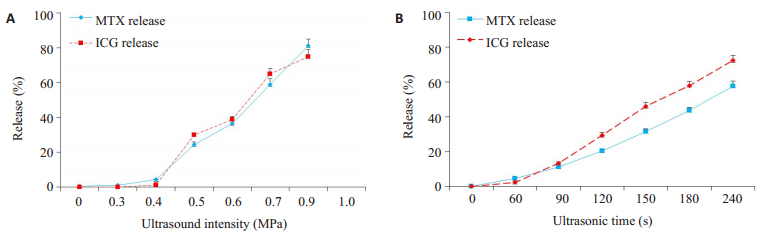
|
图 2 低频超声作用下iRGD-MTX-ICG-ELIP释放药物曲线 Figure 2 Drug release curve of iRGD-MTX-ICG-ELIP under low frequency ultrasound. A: Drug release rate changes with ultrasonic intensity; B: Drug release rate changes with ultrasonic time. |
激光共聚焦下观察到DAPI所染的HUVECs细胞核呈蓝色,包载了ICG的iRGD-MTX-ICG-ELIP和MTX-ICG-ELIP进入HUVECs细胞质后呈红色,而iRGD-MTX-ICG-ELIP组的红色荧光强度明显强于MTX-ICG-ELIP组(图 3A)。流式细胞实验得出HUVECs对iRGD-MTX-ICG-ELIP的摄取率比对MTX-ICG-ELIP和游离的ICG药物的摄取高1.89倍和3.31倍,差异有统计学意义(F=4434.61,P < 0.05,图 3B、3C)。
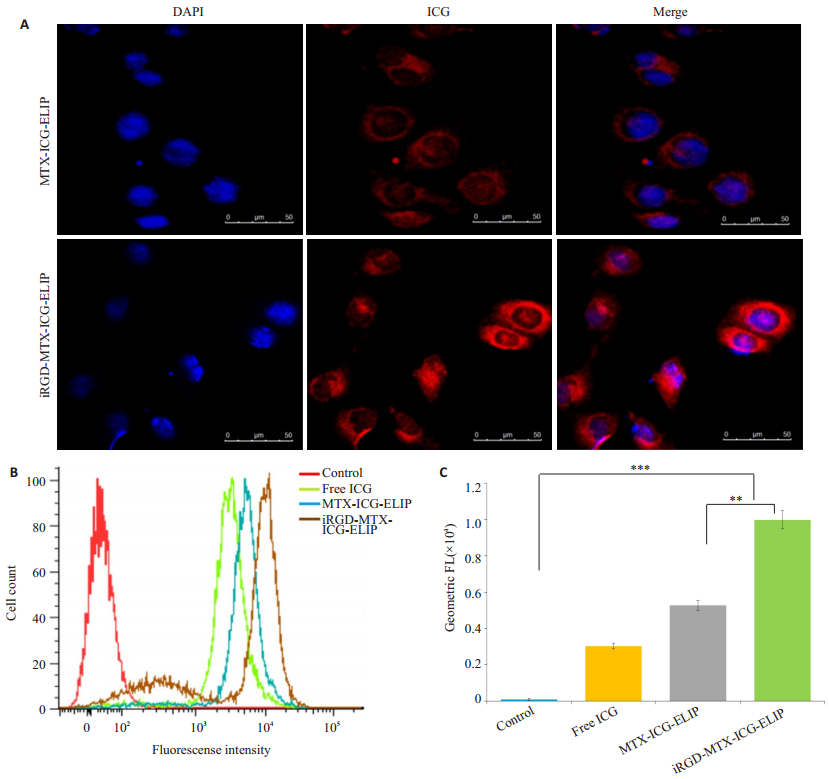
|
图 3 iRGD-MTX-ICG-ELIP在HUVECs的摄取情况 Figure 3 Uptake of MTX-ICG-ELIP and iRGD-MTX-ICG-ELIP in HUVECs. A: MTX-ICG-ELIP and iRGD-MTX-ICG-ELIP uptake in HUVECs under confocal laser microscope (scale bar: 50 μm); B: Flow cytometry showing liposome uptake in each group; C: Fluorescence intensity of HUVECs in each group compared by geometric mean method (**P < 0.05, ***P < 0.05). |
在RA小鼠模型的构建过程中,正常小鼠(图 4A)初次免疫26 d即可观察到关节炎的发病症状,第48天左右关节红肿到达较高峰(图 4B)。RA小鼠活体近红外成像实验结果显示:iRGD靶向的载药声学脂质体组的RA小鼠红肿关节的荧光明显强于非靶向载药声学脂质体(图 4C、D)。
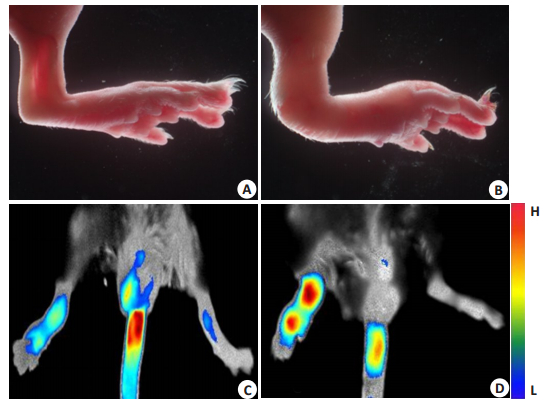
|
图 4 RA小鼠模型构建及近红外活体成像 Figure 4 RA mouse model and near-infrared imaging in vivo. A: Normal joint; B: Inflamed paw of RA mouse model; C: Distribution of MTX-ICG-ELIP in RA mouse model; D: Distribution of iRGD-MTX-ICG-ELIP in RA mouse model. |
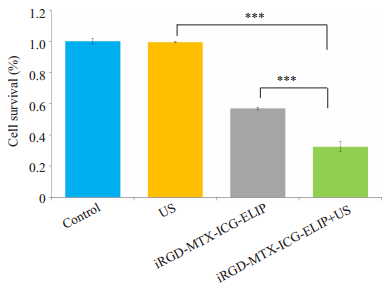
通过观察对照组及各处理组抑制MH7A增殖实验发现,对照组,单纯超声组,iRGD-MTX-ICG-ELIP,iRGD-MTX-ICG-ELIP+超声组,各组间MH7A存活率分别为(100±1.77)%、(99.42±0.34)%、(56.99±0.67)%、(32.49±3.04)%,MTX-ICG-ELIP+超声组抑制MH7A增殖作用最强,除对照组与单纯超声组外,余各组间均差异有统计学意义(F=1324.379,P < 0.05,图 5)。
|
图 5 CCK8检测各组抑制MH7A存活率 Figure 5 Survival rate of MH7A cells in each group (***P < 0.05). |
RA的病理进展与肿瘤的发展相似,RA发生后关节滑膜细胞呈肿瘤样增殖,凋亡减少[13]。由于RA的反复发作,增厚明显的滑膜细胞层可使关节面相互粘连,关节病变加重,最终导致关节骨和软骨破坏,影响关节的活动,因而抑制滑膜细胞增殖是治疗RA的关键。在众多RA治疗的药物中,作为一线用药的MTX [14-15]一方面可以通过抗叶酸代谢达到抑制关节滑膜细胞增殖的作用,另一方面通过抑制细胞的活化和增殖来发挥免疫抑制作用。体内外实验[16-17]均证实MTX对滑膜细胞具有良好的抑制作用,因而本研究选用MTX作为治疗RA的基石药物。
RA发病的中心病理环节涉及关节滑膜血管新生和血管翳的形成以及它们对关节的侵蚀[18]。这些新生血管内皮细胞[19]高水平表达整合素αvβ3受体,而iRGD [20](CRGDKGPDC)是一种能够靶向αvβ3受体并加速药物传递的环形穿膜肽,可通过对其末端修饰耦联不同的基团实现与纳米载药体系的结合[21]。本研究将iRGD肽连接于纳米载药脂质体表面,制备了靶向αvβ3受体的iRGD-MTX-ICG-ELIP,通过细胞摄取实验(图 3)示血管内皮细胞对iRGD-MTX-ICG-ELIP的摄取效率较非靶向组和游离ICG组高1.89倍。RA小鼠近红外活体成像更直观地显示了iRGD-MTX-ICG-ELIP较非靶组在小鼠病变关节的荧光强度更强,说明其在病变关节聚集的更多,对病变关节的靶向性更强,其有助于实现RA的分子成像与药物靶向释放。
声学脂质体[22-24]是一种具有声学响应性的纳米载药体系,其保留了普通脂质体传递药物的特点,并且能在低频超声作用下通过其内含空化壳,在脂质体表面产生空化效应,在提高脂质体磷脂膜的通透性下更好地促进药物释放,增加药物向组织的渗透。本研究通过薄膜水化及冷冻干燥法制备的载药声学脂质体iRGDMTX-ICG-ELIP粒径小,均一性好,经超滤分离后测得其包裹的水溶性药物MTX和ICG的包封率均达60%以上,具有较好的药物包载量,为RA病变关节药物浓度的提高提供了基础。而其联合超声控释药物实验显示了iRGD-MTX-ICG-ELIP能在低频超声作用下立即释放药物,且药物释放量随超声作用强度和作用时间延长而增多,表明其具有良好的声学响应性。而iRGD-MTXICG-ELIP将MTX包载入磷脂膜内,既优化了MTX在体内组织的分布、降低了全身用药的毒副作用[25-26],又使药物能在低频超声作用下定点释放[27]。本研究体外抑制MH7A增殖实验结果示iRGD-MTX-ICG-ELIP能够抑制MH7A的增殖,而且其联合超声的抑制作用要显著高于其他组,抑制效果约为未联合超声组的1.7倍(56.99%/32.49%),说明iRGD-MTX-ICG-ELIP在超声作用下进一步增强了对MH7A的抑制。此外iRGDMTX-ICG-ELIP又将可用于近红外成像的荧光探针ICG [28-30]包裹于此体系中,用以实现目标载体在RA病变关节靶向聚集的可视化,并指导其释放药物,本研究将iRGD-MTX-ICG-ELIP通过尾静脉注射后发现其可用于RA小鼠关节的荧光成像,能为RA的治疗提供影像引导。
综上所述,本研究所构建的多功能纳米载体iRGDMTX-ICG-ELIP,对RA关节病变不但具有良好的靶向性和荧光成像潜能,而且其联合超声在体外实现了良好的治疗效果,为后续RA体内动物治疗及近红外成像实验研究奠定了基础,为诊疗一体化提供了一种新思路。
| [1] | Scott DL, Wolfe F. Rheumatoid arthritis[J]. Lancet, 2010, 376(9746): 1094-108. DOI: 10.1016/S0140-6736(10)60826-4. |
| [2] | Barhamain AS, Magliah RF, Shaheen MH, et al. The journey of rheumatoid arthritis patients:a review of reported lag times from the onset of symptoms[J]. Open Access Rheumatology:Research and Reviews, 2017, 9(3): 139-50. |
| [3] | Ichihara H, Yamasaki S, Hino M, et al. Therapeutic effects of hybrid liposomes with downregulation of inflammatory cytokine for model mice of rheumatoid arthritis in vivo[J]. Bioorg Med Chem Lett, 2015, 25(13): 2686-9. DOI: 10.1016/j.bmcl.2015.04.083. |
| [4] | Tins BJ, Butler R. Imaging in rheumatology:reconciling radiology and rheumatology[J]. Insights Imaging, 2013, 4(6): 799-810. DOI: 10.1007/s13244-013-0293-1. |
| [5] | Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs[J]. Ann Rheum Dis, 2010, 69(6): 964-75. DOI: 10.1136/ard.2009.126532. |
| [6] | Morgan C, Lunt M, Brightwell H, et al. Contribution of patient related differences to multidrug resistance in rheumatoid arthritis[J]. Ann Rheum Dis, 2003, 62(1): 15-9. DOI: 10.1136/ard.62.1.15. |
| [7] | Qiu Q, Huang J, Lin Y, et al. Polymorphisms and pharmacogenomics for the toxicity of methotrexate monotherapy in patients with rheumatoid arthritis:A systematic review and meta-analysis[J]. Medicine, 2017, 96(11): e6337. DOI: 10.1097/MD.0000000000006337. |
| [8] | Rahman M, Sarwar B, Sharma G, et al. Lipid-based vesicular nanocargoes as nanotherapeutic targets for the effective management of rheumatoid arthritis[J]. Recent Pat Antiinfect Drug Discov, 2016, 11(4): 3-15. |
| [9] | Kapoor B, Singh SK, Gulati M, et al. Application of liposomes in treatment of rheumatoid arthritis:quo vadis[J]. Scientific World Journal, 2014, 4: 978351. |
| [10] | Huang SL. Liposomes in ultrasonic drug and gene delivery[J]. Adv Drug Deliv Rev, 2008, 60(10): 1167-76. DOI: 10.1016/j.addr.2008.03.003. |
| [11] | Lin CY, Javadi M, Belnap DM, et al. Ultrasound sensitive eLiposomes containing doxorubicin for drug targeting therapy[J]. Nanomedicine, 2014, 10(1): 67-76. DOI: 10.1016/j.nano.2013.06.011. |
| [12] | Nahire R, Paul S, Scott MD, et al. Ultrasound enhanced matrix metalloproteinase-9 triggered release of contents from echogenic liposomes[J]. Mol Pharm, 2012, 9(9): 2554-64. DOI: 10.1021/mp300165s. |
| [13] | Hitchcock KE, Caudell DN, Sutton JT, et al. Ultrasound-enhanced delivery of targeted echogenic liposomes in a novel ex vivo mouse aorta model[J]. J Control Release, 2010, 144(3): 288-95. DOI: 10.1016/j.jconrel.2010.02.030. |
| [14] | Thairu N, Kiriakidis S, Dawson P, et al. Angiogenesis as a therapeutic target in arthritis in 2011:learning the lessons of the colorectal cancer experience[J]. Angiogenesis, 2011, 14(3): 223-34. DOI: 10.1007/s10456-011-9208-2. |
| [15] | Chan ES, Cronstein BN. Methotrexate-how does it really work[J]. Nat Rev Rheumatol, 2010, 6(3): 175-8. DOI: 10.1038/nrrheum.2010.5. |
| [16] | Saag KG, Teng GG, Patkar NM, et al. American college of rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis[J]. Arthritis Rheum, 2008, 59(6): 762-84. DOI: 10.1002/(ISSN)1529-0131. |
| [17] | Gottschalk O, Metz P, Dao Trong ML, et al. Therapeutic effect of methotrexate encapsulated in cationic liposomes (EndoMTX) in comparison to free methotrexate in an antigen-induced arthritis study in vivo[J]. Scand J Rheumatol, 2015, 44(6): 456-63. DOI: 10.3109/03009742.2015.1030448. |
| [18] | Nakaya H, Shimizu T, Isobe K, et al. Microbubble-enhanced ultrasound exposure promotes uptake of methotrexate into synovial cells and enhanced antiinflammatory effects in the knees of rabbits with antigen-induced arthritis[J]. Arthritis Rheum, 2005, 52(8): 2559-66. DOI: 10.1002/(ISSN)1529-0131. |
| [19] | Foster W, Shantsila E, Carruthers D, et al. Circulating endothelial cells and rheumatoid arthritis:relationship with plasma markers of endothelial damage/dysfunction[J]. Rheumatology (Oxford), 2009, 48(3): 285-8. |
| [20] | Atehortúa L, Rojas M, Vásquez GM, et al. Endothelial alterations in systemic lupus erythematosus and rheumatoid arthritis:potential effect of monocyte interaction[J]. Mediators Inflamm, 2017: 9680729. DOI: 10.1155/2017/9680729. |
| [21] | Liu Y, Ji M, Wong MK, et al. Enhanced therapeutic efficacy of iRGD-Conjugated crosslinked multilayer liposomes for drug delivery[J]. Biomed Res Int, 2013: 378380. |
| [22] | Yan F, Wu H, Liu H, et al. Molecular imaging-guided photothermal/photodynamic therapy against tumor by iRGD-modified indocyanine green nanoparticles[J]. J Control Release, 2016, 224(13): 217-28. |
| [23] | Buchanan KD, Huang SL, Kim H, et al. Encapsulation of NF-kappa B decoy oligonucleotides within echogenic liposomes and ultrasound-triggered release[J]. J Control Release, 2010, 141(2): 193-8. DOI: 10.1016/j.jconrel.2009.09.017. |
| [24] | Radhakrishnan K, Haworth KJ, Peng T, et al. Loss of echogenicity and onset of cavitation from echogenic liposomes:pulse repetition frequency independence[J]. Ultrasound Med Biol, 2015, 41(1): 208-21. DOI: 10.1016/j.ultrasmedbio.2014.08.021. |
| [25] | Rizzitelli S, Giustetto P, Cutrin JC, et al. Sonosensitivetheranostic liposomes for preclinical in vivo MRI-guided visualization of doxorubicin release stimulated by pulsed low intensity non-focused ultrasound[J]. J Control Release, 2015, 202(5): 21-30. |
| [26] | Prabhu P, Shetty R, Koland M, et al. Investigation of nano lipid vesicles of methotrexate for anti-rheumatoid activity[J]. Int J Nanomedicine, 2012, 7(1): 177-86. |
| [27] | Wang X, Liu P, Yang WX, et al. Microbubbles coupled to methotrexate-loaded liposomes for ultrasound-mediated delivery of methotrexate across the blood-brain barrier[J]. Int J Nanomedicine, 2014, 9(3): 4899-909. |
| [28] | Smith DA, Vaidya SS, Kopechek JA, et al. Ultrasound-triggered release of recombinant tissue-type plasminogen activator from echogenic liposomes[J]. Ultrasound Med Biol, 2010, 36(1): 145-57. DOI: 10.1016/j.ultrasmedbio.2009.08.009. |
| [29] | Kraft JC, Ho RJ. Interactions of indocyanine green and lipid in enhancing Near-Infrared fluorescence properties:the basis for near-infrared imaging in vivo[J]. Biochemistry, 2014, 53(8): 1275-83. DOI: 10.1021/bi500021j. |
| [30] | Tansi FL, Rueger R, Rabenhold M, et al. Fluorescence-quenching of a liposomal-encapsulated near-infrared fluorophore as a tool for in vivo optical imaging[J]. J Vis Exp, 2015(95): 52136. |
| [31] | Onishi S, Sakane M, Toshinori T, et al. Near-infrared fluorescence imaging with indocyanine green-lactosomes in a mouse modern rheumatology model of rheumatoid arthritis[J]. Mod Rheumatol, 2016, 5(4): 1-6. |
 2017, Vol. 37
2017, Vol. 37

