Spontaneous coronary artery dissection (SCAD) is an uncommon condition that is often misdiagnosed as acute coronary syndrome[1, 2]. Although a young age and a female gender were reportedly associated with SCAD, the etiology and pathogenesis of SCAD are not fully understood[3]. Here we report a rare case of SCAD in a seemingly healthy postmenopausal woman who developed acute myocardial infarction and subsequent ventricular aneurysm after emotional stress. We also reviewed the important pathophysiological and epidemiological features of SCAD and the current approaches to its diagnosis and management.
CASE REPORTA 52-year-old female patient was admitted in the Department of Emergency Medicine of our hospital for sudden acute severe pressure-like substernal chest pain for 2 h following the death of her mother 2 days before. She denied having experienced any similar episodes of pain previously. The patient was a nonsmoker without a history of hypertension or diabetes mellitus, and showed a serum low-density lipoprotein (LDL-C) level of 4.07 mmol/L after admission. She attained menopause around the age of 44 years. At admission, her blood pressure was 130/80 mmHg with a pulse rate of 68 beats/min. Electrocardiography (ECG) revealed sinus rhythm and complete right bundle branch block (RBBB) with slight T-wave inversions in leads Ⅲ and aVF (Fig. 1A). Her peak level of creatine kinase MB reached 73.60 U/L (normal range, 0-24 U/L) within 24 h following the onset of chest pain, and her troponin T level was 0.619 ng/mL (normal, 0-0.014 ng/mL). The complete blood count, comprehensive metabolic panel, coagulation tests, hypothyroid function, rheumatism factor, and erythrocyte sedimentation rate were within normal ranges. The levels of high-sensitivity C-reactive protein (hsCRP) and N-terminal pro-brain natriuretic peptide (NT-proBNP) levels were 10.5 mg/L (normal, 2.1-3.0 mg/L) and 418 pg/mL (normal, 0-450 pg/mL), respectively. Our primary diagnosis was acute non-ST segment elevation myocardial infarction.
|
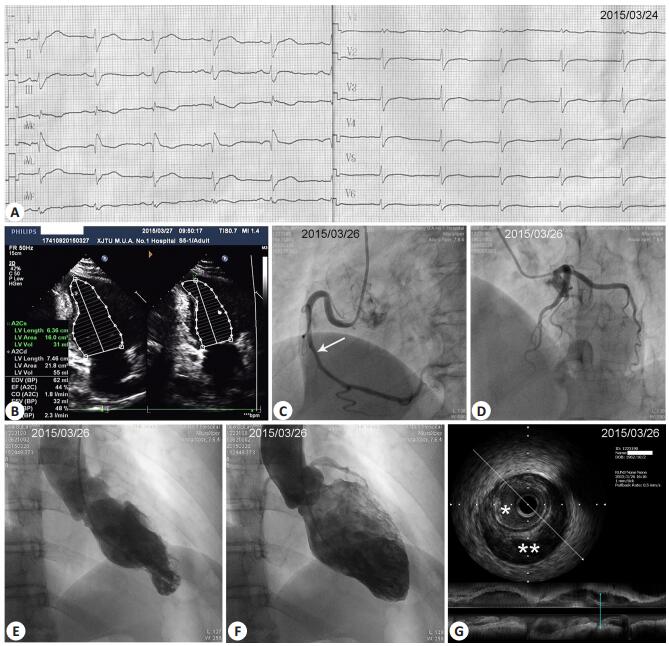
Figure 1 Results of examinations of the patients at admission. A: 12-lead electrocardiogram showing RBBB and slight T-wave inversions in leads Ⅲ and aVF; B: Echocardiogram showing localized hypokinesia of the apical-inferior wall and an apical-inferior ventricular aneurysm; C: Coronary angiography (CAG) showing 50% stenosis from the middle RCA up to the distal RCA. White arrow near the middle RCA indicates the approximate position where later intravascular ultrasound (IVUS) image was taken (shown in G); D: CAG showing normal LAD and LCX arteries; E, F: Left ventriculography showing a small left ventricular apical-inferior segment aneurysm at systole (E) or end-systole (F); G: IVUS showing an intramural hematoma that caused lumen compression. *Lumen; **Intramural hematoma. |
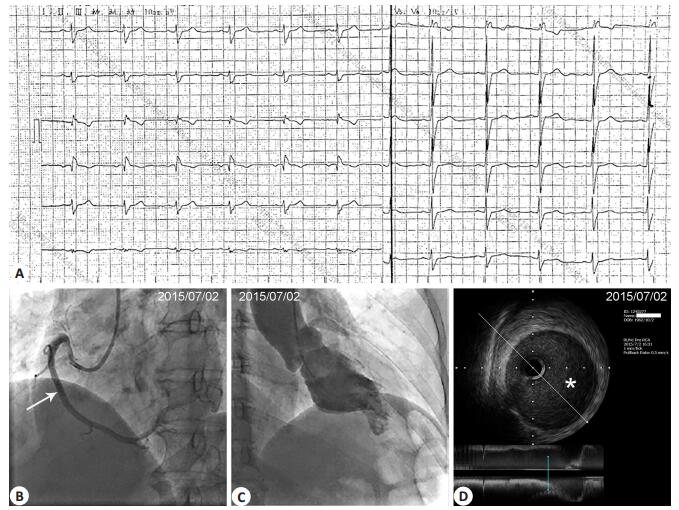
The patient was initially treated with aspirin, clopidogrel, enoxaparin, metoprolol, perindopril, and atorvastatin. Transthoracic echocardiography (TTE) showed localized hypokinesia of the apical-inferior wall and an apical-inferior ventricular aneurysm. She had an estimated ejection fraction of 48% by Simpson's method in the apical two-chamber view (Tab. 1 and Fig. 1B). Coronary angiography (CAG) revealed 50% stenosis of the middle to distal right coronary artery (RCA) with normal flow down the distal vessel (Fig. 1C). The left anterior descending (LAD) and left circumflex (LCX) arteries were both angiographically normal (Fig. 1D). On suspicion of spontaneous RCA dissection, intravascular ultrasonography (IVUS) was performed, which revealed an intramural hematoma starting from the mid RCA up to the distal RCA with luminal compromise (Fig. 1G). Left ventriculography showed a small left ventricular apical-inferior segment aneurysm at end-systole (Fig. 1E, F). Because of her stable clinical condition and the patency of the coronary artery with normal blood flow, the patient received conservative treatment with medications without additional procedures. She did not experience any further anginal episodes. At 3 months after the onset, her hsCRP and NT-proBNP returned to the normal levels with a lowered LDL-C level of 2.05 mmol/L (Tab. 1). Her ECG had no significant change compared with that at admission (Fig. 2A). CAG revealed normalization of the dissected RCA segment (Fig. 2B), and IVUS showed almost complete disappearance of the intramural hematoma (Fig. 2D). Repeat TTE and left ventriculography still showed the presence of the left ventricular apical-inferior aneurysm (Fig. 2C), but the ejection fraction (EF) measured by echocardiography recovered the normal level (78%) (Tab. 1).
| Table 1 Blood biochemical and echocardiographic examination of the patient at admission and 3 months later |
|
Figure 2 Results of examination at 3 months after the onset. A: Electrocardiogram showing RBBB without obvious changes compared with that at admission; B: CAG showing normalization of the dissected RCA. White arrow at the middle RCA indicates the approximate position where IVUS image was taken (shown in D); C: Repeat left ventriculography showing the persistence of left ventricular apical-inferior aneurysm at systole; D: IVUS showing the complete disappearance of intramural hematoma and the full recovery of the lumen size. *Lumen. |
SCAD, defined as the separation of the layers of the arterial wall with the formation of two lumens [4], is a rare condition with a high mortality rate in the acute stage. SCAD associated with emotional stress is even more infrequent. The overall prevalence of SCAD is only 0.2%-1.1% among patients undergoing CAG [4], and more than 70% of the cases occur in women often at the age ranging from 35 to 40 years [5]. The etiology and pathogenesis of SCAD remain poorly understood. The risk factors of SCAD include pregnancy, Ehlers-Danlos disease, Marfan's syndrome, intensive exercise, and cocaine abuse. Genetic predisposition is also proposed to contribute to SCAD occurrence [6], and the Mayo Clinic SCAD Registry has identified 5 familial cases of SCAD implicating both recessive and dominant modes of inheritance from 412 enrolled patients; none of the participants had other potential risk factors for SCAD.
SCAD frequently affects young women, and its occurrence in healthy postmenopausal women is rare. In the case we presented herein, the healthy woman had no known risk factors for SCAD except for a history of intense emotional stress. So far only scattered cases have been reported to document emotional stress as the trigger of SCAD [7, 8]. However, in 168 patients with SCAD prospectively evaluated, as many as 57% of the patients reported precipitating stressors preceding their SCAD event, and approximately 40% reported being emotionally stressed (e.g., death in the family, breakdown of marriage, arguments, and job stress) [9, 10]. The role of emotional stress as a causal factor of SCAD remains elusive. One possibility is that coronary arteries undergo intense spasm in response to intense emotional stress, which has been causally linked to the incidence of SCAD [11], but it remains unknown whether the vasospasm leads to dissection or the dissection itself leads to secondary vasospasm [11]. Another possible explanation is that emotional stress triggers excessive activation of the sympathetic nervous system, which may cause extensive shearing stresses on the coronary arteries to disrupt the thin-walled vasa vasorum and results in hemorrhage within the media of the artery[10].
In the case we presented herein, our initial speculation was that emotional stress caused SCAD and Takotsubo cardiomyopathy (TCM) simultaneously, which was similar with the descriptions in previous reports [4, 12]. Y-Hassan[4] proposed that TCM was a result of postischemic myocardial stunning triggered by SCAD. But in our case, repeat left ventriculography showed that the balloon-like appearance of the apical wall persisted till 3 months after the onset, in contrast to the reversible cardiomyopathy in TCM. We eventually concluded that SCAD triggered by emotional stress caused acute myocardial infarction and the development of a ventricular aneurysm. But why did 50% stenosis of RCA with normal blood flow cause myocardial infarction in this case? We propose that SCAD may firstly result in giant intramural hematoma, which led to severe coronary stenosis and acute myocardial infarction. Then the intramural hematoma extended to both end of coronary artery, and the compression of hematoma to the true lumen was reduced subsequently, which presented later as moderate lumen stenosis on CAG.
Generally, patients with SCAD have favorable long-term outcomes, and conservative therapy can be safe in the majority of cases [13]. But as a life-threatening condition, SCAD can cause early or late complications including SCAD of another vessel [14]. The treatment decision must be made based on both the clinical and angiographic factors of the individual patients [15]. Revascularization of the dissected artery should be performed with discretion after full evaluation of the patient's clinical conditions and the anatomy of the affected coronary [16]. Percutaneous coronary intervention may be unsuccessful in 35% of the cases [17]. In our case, conservative therapy resulted in a favorable outcome and the resolution of SCAD in spite of the presence of the ventricular aneurysm.
In conclusion, our case demonstrates that SCAD can occur in healthy postmenopausal women after intense emotional stress to cause acute myocardial infarction and subsequent development of a ventricular aneurysm. This case provides valuable insight into this rare disease and urges more attention to the psychological factors at the time of diagnosis in seemingly healthy women with acute chest pain.
| [1] | Saw J, Mancini GB, Humphries KH. Contemporary review on spontaneous coronary artery dissection[J]. J Am Coll Cardiol, 2016, 68(3): 297-312. DOI: 10.1016/j.jacc.2016.05.034. |
| [2] | Alfonso F, Bastante T, Garcia-Guimaraes M, et al. Spontaneous coronary artery dissection: new insights into diagnosis and treatment[J]. Coron Artery Dis, 2016, 27(8): 696-706. DOI: 10.1097/MCA.0000000000000412. |
| [3] | Afzal A, Sarmast S, Choi JW, et al. Spontaneous coronary artery dissection: a review of pathogenesis, presentations, treatment, and outcomes[J]. Rev Cardiovasc Med, 2017, 18(1): 29-36. |
| [4] | Y-Hassan S, Henareh L. Spontaneous coronary artery dissection triggered post-ischemic myocardial stunning and takotsubo syndrome: two different names for the same condition[J]. Cardiovasc Revasc Med, 2013, 14(2): 109-12. DOI: 10.1016/j.carrev.2012.11.005. |
| [5] | Alfonso F, Bastante T, Cuesta J, et al. Spontaneous coronary artery dissection: novel insights on diagnosis and management[J]. Cardiovasc Diagn Ther, 2015, 5(2): 133-40. |
| [6] | Goel K, Tweet M, Olson TM, et al. Familial spontaneous coronary artery dissection: evidence for genetic susceptibility[J]. JAMA Intern Med, 2015, 175(5): 821-6. DOI: 10.1001/jamainternmed.2014.8307. |
| [7] | Mayr A, Klug G, Jaschke W, et al. Persistent spontaneous dissection of the left anterior descending coronary artery after emotional pressure[J]. Wien Klin Wochenschr, 2010, 122(15-16): 515-7. DOI: 10.1007/s00508-010-1422-1. |
| [8] | Eugene M, Siam-Tsieu V, Pilliere R, et al. Recurrent spontaneous coronary artery dissection: unexpected evolution and major role of emotional stress[J]. Int J Cardiol, 2015: 201316-8. |
| [9] | Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes[J]. Circ Cardiovasc Interv, 2014, 7(5): 645-55. DOI: 10.1161/CIRCINTERVENTIONS.114.001760. |
| [10] | Thomas BN, Aslam S, Cullen J, et al. Spontaneous coronary artery dissection in men presenting with acute coronary syndrome, successfully managed by intravascular ultrasound-guided percutaneous coronary intervention[J]. BMJ Case Rep, 2014 Mar 7;2014. pⅱ: bcr2013009169. http://www.ncbi.nlm.nih.gov/pubmed/24717852 |
| [11] | Arrivi A, Milici C, Bock C, et al. Idiopathic, serial coronary vessels dissection in a young woman with psychological stress: a case report and review of the literature[J]. Case Rep Vasc Med, 2012, 2012: 498465. |
| [12] | Y-Hassan S, Themudo R, Maret E. Spontaneous coronary artery dissection and takotsubo syndrome: the chicken or the egg causality dilemma[J]. Catheter Cardiovasc Interv, 2017, 89(7): 1215-8. DOI: 10.1002/ccd.v89.7. |
| [13] | Y-Hassan S. Treatment strategy of spontaneous coronary artery dissection: conservative or interventional[J]. Int J Cardiol, 2015: 19182-3. |
| [14] | Rogowski S, Maeder MT, Weilenmann D, et al. Spontaneous coronary artery dissection: angiographic follow-up and long-term clinical outcome in a predominantly medically treated population[J]. Catheter Cardiovasc Interv, 2017, 89(1): 59-68. DOI: 10.1002/ccd.26383. |
| [15] | Martins JL, Afreixo V, Santos L, et al. Medical treatment or revascularisation as the best approach for spontaneous coronary artery dissection: a systematic review and meta-analysis[J]. Eur Heart J Acute Cardiovasc Care, 2017. Apr 1: 2048872617706502. http://www.ncbi.nlm.nih.gov/pubmed/28452228/ |
| [16] | Lee R, Ben-Dor I. Revascularization methods in spontaneous coronary artery dissection: a focused review[J]. Cardiovasc Revasc Med, 2017 May 12. pⅱ: S1553-8389(17)30171-9. http://www.ncbi.nlm.nih.gov/pubmed/28529093 |
| [17] | Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection[J]. Circulation, 2012, 126(5): 579-88. DOI: 10.1161/CIRCULATIONAHA.112.105718. |
 2017, Vol. 37
2017, Vol. 37
