2. Key Laboratory of Environment and Genes Related to Diseases of Ministry of Education, Xi'an Jiaotong University College of Medicine, Xi'an 710061, China
2. 西安交通大学医学院 环境与疾病相关基因教育部重点实验室,陕西 西安 710061
As a member of the thiazolidinedione (TZD) family, a class of agonists of nuclear receptor peroxisome proliferator-activated receptor (PPAR)-γ, pioglitazone (PIO) has been widely used in the treatment of diabetes. Clinical trials suggest that PIO also has potential cardiovascular benefits[1-3] possibly through amelioration of atherosclerosis [4-6] and modulation of the immune system [7-10]. Because the immune response plays a pathogenetic role in atherosclerosis [11], the antiinflammatory activity of TZDs may contribute to its cardiovascular benefits.
Accumulating evidence suggests the involvement of adaptive T cell immunity in atherogenesis. For instance, T helper 1-cells (Th1), a specific phenotype of CD4+ T cells that produce large quantities of IFN-γ, are known to stimulate the pro-atherosclerotic processes [11]. The regulatory T (Treg) cells expressing specific transcription factor Foxp3, a subpopulation of CD4+ T cells, have been found to suppress the pathogenic immune response of T helper cells against self and foreign antigens as well as to maintain T cell homeostasis [12, 13]. In animal studies, Treg cells were found to participate in the development of atherosclerosis[14-16].
Transforming growth factor-β (TGF-β) is the main cytokine produced by Treg cells and plays a crucial role in the regulation of the inflammatory process[17]. Recent studies suggest that TGF-β is a master of all T cell trades, capable not only of regulating CD4+T cell subset proliferation and balance [18, 19] but also of inhibiting the progression of the atherosclerotic plaques and maintaining the stability of the plaques in mice and human[17, 20, 21]. We hypothesized that PIO could inhibit atherosclerosis through TGF-β/Smad signaling to modulate the balance of Th1 and Treg cells. In this study, we tested this hypothesis by investigating whether the protective effect of IPO against atherogenesis is PPARγ-dependent.
MATERIALS AND METHODS AnimalsA total of 22 apolipoprotein E-deficient (ApoE-/-) mice (B6.129P2-ApoEtm1Unc strain) were purchased from Model Animal Research Center of Nanjing University (China) and kept in a temperature-controlled facility on a 12 h light/dark cycle with free access to food and water. All the mice were weaned at 4 weeks of age and fed normal chow till 6 weeks of age. The mice were then fed a high-fat diet (21% fat and 0.15% cholesterol) for 8 weeks until early atherosclerotic lesions (such as fatty streak) occurred in the root of aorta.
Animal treatmentPIO was a generous gift from Hengrui Medicine, Ltd. (Jiangsu Province, China) and was dissolved in 0.5% sodium carboxy methyl cellulose to a concentration of 10 mg/mL before use. Sixteen of the ApoE-/- mice at the age of 6 weeks were randomized into two equal groups for high-fat feeding and daily treatment with vehicle or PIO (20 mg/kg per day) by gastric gavage for 8 weeks.
Quantitative assessment of atheromatous lesions in the aortaThe aortic root of the mice was dissected under a microscope and frozen in optimal cutting temperature embedding medium for serial 6 μm cyosectioning, which covered 500 μm of the aortic root. The atheromatous lesions were detected using oil red O staining. The cross-sectional surface area of the atheromatous lesion and the vascular area were quantified.
Immunohistochemical analysis of the aorta and spleenImmunohistochemical analysis was performed for TGF-β1, TGF-β receptor Ⅱ (TGFβRⅡ), Smad3, p-Smad3 and Smad7. The aorta sections were deparaffinized, hydrated and blocked with 3% hydrogen peroxide for 15 min. After blocking with 5% bovine serum albumin (BSA) for 40 min, the sections were incubated overnight at 4 ℃ with the primary antibodies against TGF-β1 (Cell Signaling Technology, 1:100), TGFβRⅡ (Santa Cruz 1: 100), Smad3 (Abcam, 1:60), p-Smad3 (Abcam, 1:60) or Smad7 (Santa Cruz, 1: 100) individually. After incubation of the sections with horseradish peroxidase (HRP)-labeled rat IgG2a antibody (1:500, Sigma) at 37 ℃ for 30 min, the expressions of the proteins were visualized by 3, 3'-diaminobenzidine tetrahydrochloride staining. The section images were captured digitally using an Olympus BX51 imaging system and quantitatively analyzed using Image-Pro Plus 6.0 software.
The spleen of the mice was collected and frozen in optimal cutting temperature embedding medium for serial 6 μm cryosectioning. Immunohistochemical analysis was performed with antibodies against IFN-γ (BD PharMingen 1:100) and Foxp3 (eBioscience 1:200) using 6 spleen sections per mouse to estimate the percentages of Th1 and Treg cells in the spleen.
The percentages of the total area of the immunohistochemically positive cells were assessed using a microscopy image analysis system. A total of 3 fields were randomly chosen and analyzed at 200× or 400 × magnification.
Serum TGF-β1 measurementBlood samples (2 mL) were collected from the sacrificed mice and placed at 37 ℃ for 1 h and then at 4 ℃ for 3 h. Serum was obtained by centrifugation of the coagulated blood sample at 2500 r/min for 10 min and stored at -20 ℃ before analysis. The level of circulating TGF-β1 in 3 μL of the serum was measured using an enzymelinked immunosorbent assay (ELISA) kit (Bender Med Systems) according to the manufacturer's instructions.
Cell cultureTo examine whether the effect of PIO on Th1 and Treg is PPAR-γ dependent, we used primary cultured splenocytes obtained from the remaining 6 mice. Briefly, the splenocytes were isolated with a nylon cell strainer, and the red blood cells was lysed and subsequently washed. The splenocytes (1.5×106/mL) were cultured in RPMI 1640 medium (Gibco-BRL) supplemented with 10% fetal bovine serum (Gibco-BRL) and 1% penicillin-streptomycin (North China Pharmaceutical Co., Ltd) at 37 ℃ in 5% CO2. In all cultured splenocytes, 2 μg/mL (final concentration) of oxidized low-density lipoprotein (oxLDL) was added for antigenic stimulation. PIO at the final concentration of 2 or 20 nmol/mL was added in the cell culture, and the cells were incubated for 12 h before analysis. In another group, the splenocytes were incubated in the presence of both PIO (2 or 20 nmol/mL) and 5 nmol/mL of GW9662, a PPARγ antagonist (ALEXIS Biochemicals).
Quantitative real-time PCRQuantitative real-time PCR was performed to evaluate the mRNA expressions of IFN-γ and Foxp3 in the cultured splenocytes. Briefly, the total RNA was extracted from the splenocytes using RNA-fast kit (Fastgen, China) following the manufacturer's instructions. cDNAs were synthesized from 2 μL of the total RNA. Quantitative analysis of mRNA expression was carried out in a total volume of 25 μL using SYBR®PrimeScriptTM RT-PCR Kit (Takara BIO, Otsu, Shiga, Japan) in the iQ5TM multicolor real-time PCR detection system (Bio-Rad, USA). PCR amplification of the target mRNAs was carried out through 40 thermal cycles of denaturing at 95 ℃ for 5 s, annealing at 58.5 ℃ for 20 s, and elongation at 72 ℃ for 10 s. The primers used for the amplifications are shown in Tab. 1. The relative expression level of the target mRNAs was calculated using the 2-ΔΔCT method with GAPDH as the reference gene for normalization.
| Table 1 Primer sequences for amplification of the target genes |
The cultured splenocytes were stained with FITClabeled anti-CD4 (BD PharMingen) and PE-labeled IFN-g (BD PharMingen) to determine the presence of CD4+ IFN-γ+ cells. FITC-labeled rat IgG2b and PElabeled rat IgG1 (BD PharMingen) were used as the isotypic control.
CD4+CD25+Foxp3+ cells were detected using Foxp3 intracellular staining using mouse regulatory T cell staining kit (eBioscience) following the manufacturer's protocol. Briefly, the cells were counterstained with FITC-labeled anti-CD4 (RM4-5) and PE-labeled anti-CD25 (PC61.5). After incubation for 30 min, the cells were fixed, washed and resuspended in a permeabilization solution. The permeabilized cells were stained with APC-labeled anti-Foxp3 (FJK-16s). FITClabeled rat IgG2b, PE-labeled rat IgG1 and APClabeled rat IgG2a were used as the isotypic control. The stained cells were analyzed on a FACScan flow cytometer using CellQuest software (Becton Dickinson).
Statistical analysisThe results are expressed as Mean±SE. One-way analysis of variance (ANOVA) was used to compare the difference among the groups. A P value less than 0.05 was considered to indicate a statistically significant difference. All the statistical analyses were performed using SigmaStat3.1 software.
RESULTS PIO ameliorated atheromatous lesions in miceFeeding a high-fat and high-cholesterol diet for 8 weeks induced atherosclerotic lesions in the aortic root in all the mice. Treatment of the mice with PIO significantly reduced the areas of the atheromatous lesions as compared with areas in the control mice[(5.63±0.54)% vs (11.5 ± 1.43)%, P < 0.05; Fig. 1], indicating that PIO could ameliorate atheromatous lesions in ApoE-/- mice with high-fat feeding.
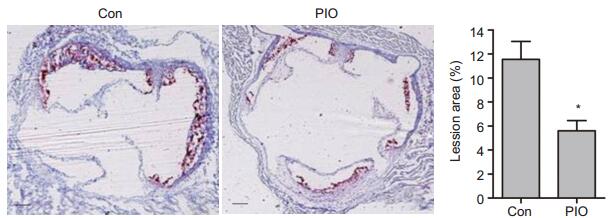
|
Figure 1 Oil red O staining and quantitative analysis of atheromatous lesions in the aorta of ApoE-/- mice (Original magnification: ×400). Scale bar: 500 μm. *P < 0.05 vs Con. |
Immunohistochemical staining showed that TGF-β1, TGFβRⅡ, Smad3, p-Smad3 (the activated form of Smad3) and the negative regulatory factor Smad7 exhibited different expression patterns in the atheromatous lesions in PIO-treated and the control mice. Compared with the control mice, the mice with PIO treatment showed significantly increased expressions of TGFβ1[(36.8 ± 10.2)% vs (28.4 ± 7.25)%, P < 0.01], TGFβRⅡ[(45.0±11.3)% vs (32.6±9.35)%, P < 0.05) and p-Smad3[(24.9 ± 9.58)% vs (19.3 ± 5.99)%, P < 0.05] (Fig. 2) and significantly decreased expression of Smad7 [(16.7±7.58)% vs (21.8±5.64)%, P < 0.05]. Furthermore, the results of ELISA also demonstrated significantly elevated serum level of TGF-β1 in PIO-treated mice as compared with that in the control mice (10.54±2.44 vs 4.80±1.83 ng/mL, P < 0.05).
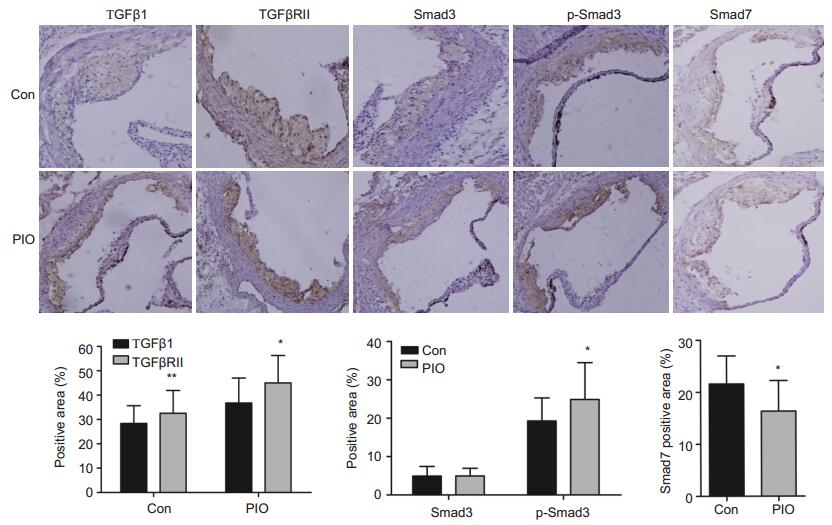
|
Figure 2 Immunohistochemical analysis of TGFβ1-signaling pathway in the aortic lesions of ApoE-/- mice (×200). *P < 0.05, **P < 0.01 vs Con. |
To determine the alternations of the numbers of Th1 and Treg cells, the expression levels of IFN-γ+ cell and Foxp3+ cells in the spleen of ApoE-/- mice with atheromatous lesions were examined by immunohistochemical analysis. As shown in Fig. 3, the number of IFN-γ+ splenocytes decreased (122±21.9/mm2 vs 221 ± 54.8/mm2, P < 0.05) and Foxp3+ splenocytes increased (260.4±33.2/mm2 vs 71.7±7.73/mm2, P < 0.01) significantly in the mice with PIO treatment in comparison with those in control mice.
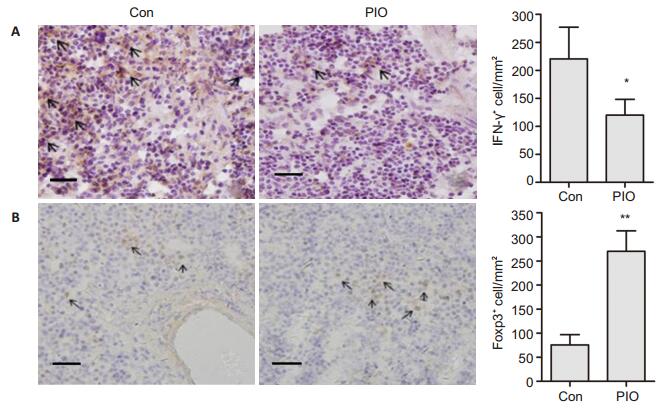
|
Figure 3 PIO decreased IFN-γ+ splenocytes and increased Foxp3+ splenocytes in spleen of ApoE-/- mice. The expression levels of IFN-γ+ cells (A) and Foxp3+ cells (B) were detected by immunohistochemical staining (×400) and quantified accordingly. Scale bar: 500 μm. *P < 0.05, **P < 0.01 vs Con. |
To examine whether the effect of PIO on Th1 and Treg cells was PPAR-γ-dependent, we detected mRNA levels of IFN-γ and foxp3 in primary cultured splenocytes isolated from ApoE-/- mice with atheromatous lesions using RT-PCR. Compared with the cells in the control group, the splenocytes incubated with 2 and 20 nmol/ mL PIO showed obvious down-regulation of IFN-γ mRNA by 40% (P < 0.05) and 70% (P < 0.05), respectively (Fig. 4A); the mRNA level of Foxp3 in the splenocytes incubated with 2 and 20 nmol/mL PIO was up-regulated by 2 folds (P < 0.05) and 2.6 folds (P < 0.01), respectively (Fig. 4B). In addition, treatment of the cells with the PPARγ antagonist GW9662 abolished the effect of PIO on IFN-γ and foxp3 mRNA expressions.
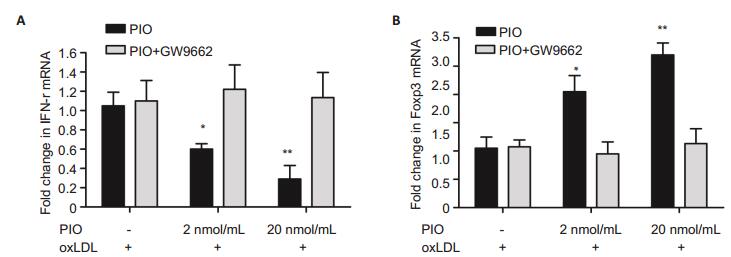
|
Figure 4 PIO decreased IFN-γ mRNA (A) and increased foxp3 mRNA (B) in cultured splenocytes as determined by RT-PCR. *P < 0.05, **P < 0.01 vs cells treated with oxLDL but not PIO. |
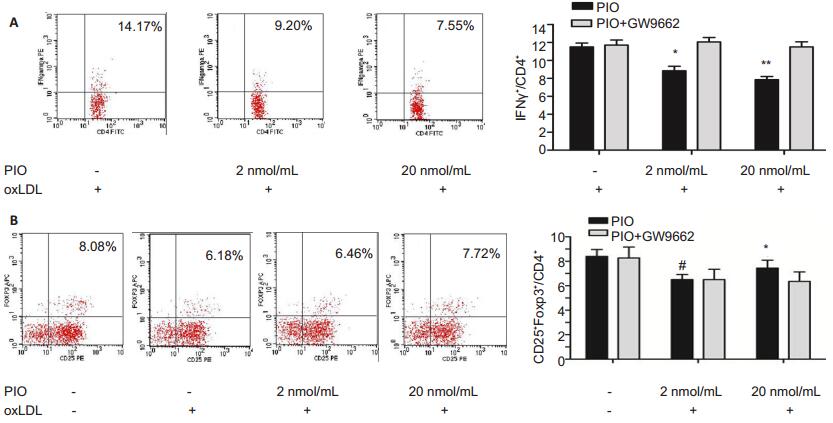
The cultured splencoytes were further analyzed by flow cytometry to estimate the percentage of IFN-γ+ and CD25+Foxp3+ cells in the CD4+ cells. As shown in Fig. 5, compared with that in the control group[(11.4±0.52)%], the percentages of CD4+ IFN-γ+ cells was decreased significantly in the splenocytes treated with 2 and 20 nmol/mL PIO [(8.68 ± 0.46)% and (7.55 ± 0.41)%, respectively, P < 0.01). The percentage of CD4+ CD25+ Foxp3+ cells was (8.19 ± 0.31)% in the splenocytes without any stimulation, but was reduced significantly to (6.31 ± 0.16)% (P < 0.05) after oxLDL stimulation; the percentage of CD4+ CD25+ Foxp3+ cells markedly increased in oxLDL-stimulated splenocytes following treatment with PIO at 2 and 20 nmol/mL[(6.73±0.22)% and (7.32 ± 0.28)%, respectively, P < 0.05]. However, co-incubation with GW9662 completely abolished the effects of PIO on CD4+ IFN-γ+ cells and CD4+ CD25+ Foxp3+ cells were, suggesting the crucial role of PPARγ in the progression of atheromatous lesions.
|
Figure 5 PIO decreased the CD4+IFN-γ+ cells (A) and increased CD4+CD25+Foxp3+ cells (B) in cultured splenocytes as detected by flow cytometry. *P < 0.05, **P < 0.01 vs cells treated with oxLDL but not PIO. #P < 0.05 vs cells treated with neither PIO nor oxLDL. |
We found that in ApoE-/- mice with high-fat feeding, PIO treatment obviously lessened aortic atheromatous lesions, increased serum TGFβ1 level as well as TGFβ 1, TGFβRⅡ and p-Smad3 expressions in the lesions, and decreased IFN-γ+ cells and increased Foxp3+ cells in the spleen. In cultured splenocytes from ApoE-/- mice, PIO treatment significantly decreased the expression level of IFN-γ mRNA, increased Foxp3 mRNA expression and increased the percentages of CD4+ IFN-γ+ cells and CD4+ CD25+ Foxp3+, demonstrating a definite effect of PIO on Th1 and Treg cell populations. Application of the PPARγ antagonist GW9662 totally abolished such effects of PIO on the cultured splenocytes, suggesting that the effect of PIO on Th1 and Treg cells was PPARγ-dependent.
Our finding that PIO ameliorates atheromatous lesions in mice is consistent with the results of previous animal and human studies[3, 22, 23]. Game et al reported that PIO treatment for 12 weeks could reduce atherosclerotic lesions in mice[22]. In patients with type 2 diabetes, a 18-month treatment with PIO was found to arrest the progression of carotid artery intima-media thickening [23] and coronary atherosclerosis [3] as compared with glimepiride treatment.
IFN-γ produced by Th1 cells, the predominant cells in adaptive immunity, is known to be involved in the atherosclerotic process [11]. We found that PIO treatment significantly decreased IFN-γ+ and increased Foxp3+ cell percentages in the spleen of mice, and in cultured splenocytes, PIO treatment decreased the expression IFN-γ mRNA and increased the expression of Foxp3 mRNA. Flow cytometry showed increased percentage of CD4+IFN-γ+ cells and CD4+CD25+Foxp3+ following PIO treatment. Taken together, these results suggest that PIO might be involved in adaptive T cell immunity through modulation of Treg and Th1 cell populations. Our hypothesis is also supported by the finding by Housley et al that PPAR-γ activation could enhance Treg generation both in vivo and in vitro[24]. Although TZDs are activators of the nuclear receptor PPAR-γ, they might inhibit atherosclerosis through both PPARγ-dependent and PPARγ-independent mechanisms [25, 26]. Substantial evidences suggest that T cells express PPAR-γ and that PPAR-γ activation inhibits the helper T cell responses [27]. Our finding that GW9662 inhibited PIOinduced changes in Treg and Th1 cell populations indicates a PPAR-γ dependence underlying the antiatherosclerotic effect of PIO.
The interaction between TGF-β signaling and PPAR-γ is well established[28, 29]. We found that in PIO treatment of the mice significantly increased TGFβ1, TGFβⅡ and p-Smad3 expressions and decreased Smad7 expression in the aortic atheromatous lesions and also increased serum TGFβ1 levels. TGF-β is known to inhibit IFN-γ production and the differentiation of both Th1 and Th2 cells [30]. TGF-β together with PPAR-γ agonists may induce Foxp3 expression in naïve CD4+ CD25-Foxp3- T cells to result in conversion of these cells into functional Tregs [31]. A recent study by Lievens et al showed that loss of TGFβRⅡ signaling in dendritic cells provoked uncontrolled effector T cell activation and resulted in increased atherosclerosis [32]. All these evidences suggest that TGFβ/Smad signaling may mediate the anti-atherosclerotic effect of PIO, probably by modulating the Th1 and Treg cells.
ConclusionPIO treatment can ameliorate the aortic atheromatous lesions in ApoE-/- mice with high-fat feeding possibly through through the TGF-β/Smad signaling pathway and PPAR-γ-dependent modulation of Th1 and Treg cell populations. More studies are needed to further understand the mechanisms underlying the antiatherosclerotic effect of PIO.
| [1] | Dormandy JA, Charbonnel B, Eckland D J, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial[J]. Lancet, 2005, 366(9493): 1279-89. DOI: 10.1016/S0140-6736(05)67528-9. |
| [2] | Lincoff AM, Wolski K, Nicholls SJ, et al. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials[J]. JAMA, 2007, 298(10): 1180-8. DOI: 10.1001/jama.298.10.1180. |
| [3] | Nissen SE, Nicholls SJ, Wolski K, et al. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial[J]. JAMA, 2008, 299(13): 1561-73. DOI: 10.1001/jama.299.13.1561. |
| [4] | Saremi A, Schwenke DC, Buchanan TA, et al. Pioglitazone slows progression of atherosclerosis in prediabetes independent of changes in cardiovascular risk factors[J]. Arterioscler Thromb Vasc Biol, 2013, 33(2): 393-9. DOI: 10.1161/ATVBAHA.112.300346. |
| [5] | Hasegawa H, Takano H, and Komuro I. Therapeutic implications of PPARgamma in cardiovascular diseases[J]. PPAR Res, 2010; 2010. pii: 876049. doi: 10.1155/2010/876049. Epub 2010 Aug 12. http://www.ncbi.nlm.nih.gov/pubmed/20814542 |
| [6] | Subramanian V, Golledge J, Ijaz T, et al. Pioglitazone-induced reductions in atherosclerosis occur via smooth muscle cell-specific interaction with PPARgamma[J]. Circ Res, 2010, 107(8): 953-8. DOI: 10.1161/CIRCRESAHA.110.219089. |
| [7] | Satoh N, Shimatsu A, Himeno A, et al. Unbalanced M1/M2 phenotype of peripheral blood monocytes in obese diabetic patients: effect of pioglitazone[J]. Diabetes Care, 2010, 33(1): e7. DOI: 10.2337/dc09-1315. |
| [8] | Yuan Z, Liu Y, Zhang J., et al. Peroxisome proliferation-activated receptor-gamma ligands ameliorate experimental autoimmune myocarditis[J]. Cardiovasc Res, 2003, 59(3): 685-94. DOI: 10.1016/S0008-6363(03)00457-7. |
| [9] | Minge CE, Ryan NK, Van Der Hoek KH, et al. Troglitazone regulates peroxisome proliferator-activated receptors and inducible nitric oxide synthase in murine ovarian macrophages[J]. Biol Reprod, 2006, 74(1): 53-60. |
| [10] | Hasegawa H, Takano H, Zou Y, et al. Pioglitazone, a peroxisome proliferator-activated receptor gamma activator, ameliorates experimental autoimmune myocarditis by modulating Th1/Th2 balance[J]. J Mol Cell Cardiol, 2005, 38(2): 25-65. |
| [11] | Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword[J]. Nat Rev Immunol, 2006, 6(7): 508-19. DOI: 10.1038/nri1882. |
| [12] | Ait-Oufella H, Salomon BL, Potteaux S, et al. Natural regulatory T cells control the development of atherosclerosis in mice[J]. Nat Med, 2006, 12(2): 178-80. DOI: 10.1038/nm1343. |
| [13] | Foks AC, Lichtman AH, Kuiper J. Treating atherosclerosis with regulatory T cells[J]. Arterioscler Thromb Vasc Biol, 2015, 35(2): 280-7. DOI: 10.1161/ATVBAHA.114.303568. |
| [14] | Mor A, Planer D, Luboshits G, et al. Role of naturally occurring CD4+ CD25+ regulatory T cells in experimental atherosclerosis[J]. Arterioscler Thromb Vasc Biol, 2007, 27(4): 893-900. DOI: 10.1161/01.ATV.0000259365.31469.89. |
| [15] | Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation[J]. Nat Immunol, 2008, 9(3): 239-44. DOI: 10.1038/ni1572. |
| [16] | Wu C, He S, Peng Y, et al. TSLPR deficiency attenuates atherosclerotic lesion development associated with the inhibition of TH17 cells and the promotion of regulator T cells in ApoE-deficient mice[J]. J Mol Cell Cardiol, 2014, 76: 33-45. DOI: 10.1016/j.yjmcc.2014.07.003. |
| [17] | Grainger DJ. Transforming growth factor beta and atherosclerosis: so far, so good for the protective cytokine hypothesis[J]. Arterioscler Thromb Vasc Biol, 2004, 24(3): 399-404. DOI: 10.1161/01.ATV.0000114567.76772.33. |
| [18] | Li MO, Flavell RA. TGF-beta: a master of all T cell trades[J]. Cell, 2008, 134(3): 392-404. DOI: 10.1016/j.cell.2008.07.025. |
| [19] | Ouyang W, Beckett O, Ma Q, et al. Transforming growth factor-beta signaling curbs thymic negative selection promoting regulatory T cell development[J]. Immunity, 2010, 32(5): 642-53. DOI: 10.1016/j.immuni.2010.04.012. |
| [20] | Tse K, Ley K. Transforming growth factor-beta: transforming plaque to stability[J]. Eur Heart J, 2013, 34(48): 3684-6. DOI: 10.1093/eurheartj/ehs228. |
| [21] | Liao P, Liu L, Wang B, et al. Baicalin and geniposide attenuate atherosclerosis involving lipids regulation and immunoregulation in ApoE-/-mice[J]. Eur J Pharmacol, 2014, 740: 488-95. DOI: 10.1016/j.ejphar.2014.06.039. |
| [22] | Game BA, He L, Jarido V, et al. Pioglitazone inhibits connective tissue growth factor expression in advanced atherosclerotic plaques in low-density lipoprotein receptor-deficient mice[J]. Atherosclerosis, 2007, 192(1): 85-91. DOI: 10.1016/j.atherosclerosis.2006.06.025. |
| [23] | Mazzone T, Meyer PM, Feinstein SB, et al. Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes: a randomized trial[J]. JAMA, 2006, 296(21): 2572-81. DOI: 10.1001/jama.296.21.joc60158. |
| [24] | Housley WJ., O'Conor CA, Nichols F, et al. PPARgamma regulates retinoic acid-mediated DC induction of Tregs[J]. J Leukoc Biol, 2009, 86(2): 293-301. DOI: 10.1189/jlb.1208733. |
| [25] | Orasanu G., Ziouzenkova O, Devchand PR, et al. The peroxisome proliferator-activated receptor-gamma agonist pioglitazone represses inflammation in a peroxisome proliferator-activated receptor-alphadependent manner in vitro and in vivo in mice[J]. J Am Coll Cardiol, 2008, 52(10): 869-81. DOI: 10.1016/j.jacc.2008.04.055. |
| [26] | Wohlfert EA, Nichols FC, Nevius E, et al. Peroxisome proliferatoractivated receptor gamma (PPARgamma) and immunoregulation: enhancement of regulatory T cells through PPARgamma-dependent and -independent mechanisms[J]. J Immunol, 2007, 178(7): 4129-35. DOI: 10.4049/jimmunol.178.7.4129. |
| [27] | Clark RB, Bishop-Bailey D, Estrada-Hernandez T, et al. The nuclear receptor PPAR gamma and immunoregulation: PPAR gamma mediates inhibition of helper T cell responses[J]. J Immunol, 2000, 164(3): 1364-71. DOI: 10.4049/jimmunol.164.3.1364. |
| [28] | Gong K, Chen YF, Li P, et al. Transforming growth factor-beta inhibits myocardial PPARgamma expression in pressure overloadinduced cardiac fibrosis and remodeling in mice[J]. J Hypertens, 2011, 29(9): 1810-9. |
| [29] | Ramirez A, Ballard EN, Roman J. TGFbeta1 controls PPARgamma expression, transcriptional potential, and activity, in part, through Smad3 signaling in murine lung fibroblasts[J]. PPAR Res, 2012, 2012: 375876. |
| [30] | Chen W, Konkel J E. TGF-beta and 'adaptive' Foxp3(+) regulatory T cells[J]. J Mol Cell Biol, 2010, 2(1): 30-6. DOI: 10.1093/jmcb/mjp004. |
| [31] | Lei J, Hasegawa H, Matsumoto T, et al. Peroxisome proliferatoractivated receptor alpha and gamma agonists together with TGF-beta convert human CD4+CD25-T cells into functional Foxp3+ regulatory T cells[J]. J Immunol, 2010, 185(12): 7186-98. DOI: 10.4049/jimmunol.1001437. |
| [32] | Lievens D, Habets K L, Robertson AK, et al. Abrogated transforming growth factor beta receptor Ⅱ (TGFbetaRⅡ) signalling in dendritic cells promotes immune reactivity of T cells resulting in enhanced atherosclerosis[J]. Eur Heart J, 2013, 34(48): 3717-27. DOI: 10.1093/eurheartj/ehs106. |
 2017, Vol. 37
2017, Vol. 37
