2. 中国医学科学院北京协和医学院,北京 100730;
3. 军事医学科学院卫生学环境医学研究所心血管药物研究中心,北京 100850
2. Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing 100730, China;
3. Cardiovascular Drug Research Center, Institute of Hea1th and Environmental Medicine, Academy of Military Medical Sciences, Beijing 100850, China
CNS炎症是神经退行性变的关键因素之一[1-3],其伴随着NF-κB的核转录和磷酸化、免疫细胞的激活及apoE的上调,最终导致认知功能受损[4]。LPS是作用于toll样受体4(TLR4)并能激活其下游炎症通路的一种细菌内毒素,腹腔或脑室注射LPS可致CNS炎症并产生认知功能损伤[5, 6]。α7烟碱乙酰胆碱受体(α7nAchR)是配体门控离子通道中的家族成员,广泛表达于CNS,此受体激动剂胆碱对大鼠缺血性脑损伤具有保护效应[7],对中到重度颅脑损伤大鼠认知功能的恢复具有促进作用,能够降低脑损伤后大鼠脑部炎症反应并上调α7nAchR表达[8]。但胆碱能否抵抗LPS所致的CNS炎症反应目前还不清楚,基于此,本研究探讨胆碱对LPS所致CNS炎症及认知功能的影响,并对其可能的机制进行探讨。
1 材料和方法 1.1 主要试剂与仪器LPS(Sigma);立体定位仪(美国stolting公司);Morris水迷宫视频分析系统(北京众实迪创公司);开场实验分析系统(国产Gene & I);共聚焦成像系统(leica TCS SP95);蛋白定量试剂盒(Thermo);iba-1、p-p38、p-38、GAPDH一抗(CST);α7nAchR一抗(abcam);山羊抗兔或羊抗鼠二抗(jackson);TNF-α、IL-1β试剂盒(安徽巧伊生物工程有限公司);酶标仪(BIO-RAD);ECL试剂盒(普利莱生物公司)。
1.2 实验动物及分组8周龄SPF级雄性C57BLC/6J小鼠,体质量18~24 g,购自北京维通利华实验动物技术有限公司,许可证号:SCXK(京)2012-0001。普通动物房中预适应3 d,剔除体质量不符的小鼠。按随机数字表法将小鼠分为空白对照组、LPS组、胆碱干预组、胆碱对照组,每组18只。
1.3 侧脑室LPS注射及药物处理LPS溶于人工脑脊液,终浓度为1 μg/uL。0.2 mL 0.4%戊巴比妥麻醉小鼠后,用立体定位仪定位,以前囟为原点,向后0.5 mm,向右旁开1.0 mm,深度为2.5 mm,运用微量进样器注射LPS或人工脑脊液,注射量为2 μL,速度为2 μL/min,注射完毕停针3 min。侧脑室注射LPS之前胆碱40 mg/kg腹腔预处理3 d,每天3次(12、2、4pm),胆碱给药贯穿整个实验过程。
1.4 Morris水迷宫实验采用Morris水迷宫测定小鼠的认知功能,历时5 d。胆碱或生理盐水预处理3 d期间,每天上午8点进行4次Morris水迷宫定位航行试验,记录小鼠每天寻找水下平台的平均潜伏期,评价小鼠的学习能力;侧脑室给予LPS或人工脑脊液24 h之后进行为期60 s的空间探索试验,记录小鼠游泳速度、穿越平台次数、目标象限移动距离百分比以及停留时间。
1.5 开场试验旷场箱为40 cm×40 cm×30 cm的透明箱,含9个方格。将小鼠放于箱体中央方格内,小鼠前肢跨出中央方格时开始计时。观察并记录其5 min内横穿的格子数和站立次数,当其身体离地并双前肢离地1 cm及以上记为1次。实验间歇用酒精擦拭旷场箱,待其挥发之后进行下1只小鼠实验。
1.6 小鼠海马齿状回iba-1蛋白的表达采用免疫组织化学方法对小鼠海马齿状回iba-1的表达进行分析。选取侧脑室注射24 h后的各组小鼠,每组6只,10%水合氯醛麻醉后迅速脱颈处死,快速剥离小鼠大脑组织,以磷酸盐缓冲液(PB)灌流后4%多聚甲醛浸泡2 d固定,石蜡包埋并作20 μm冠状切片,切片脱蜡水化后抗原修复,一抗ibal工作液1: 100 4 ℃过夜孵育,滴加envision工作液,DAB显色后水洗终止显色,苏木精复染,各级酒精梯度脱水,中性树脂封片,200倍显微镜下观察Iba1标记的细胞并拍照,对切片进行定量计数。
1.7 海马TNF-α、IL-1β检测LPS注射6 h后的小鼠海马进行蛋白抽提和定量后运用ELISA试剂盒对海马TNF-α和IL-1β炎症因子进行测定,为排除人为因素造成的实验误差,进行双复孔检测。运用酶标仪测出A450值后绘制出标准曲线,统计待测样品炎症因子含量。
1.8 蛋白质印记检测LPS注射24 h后的小鼠脱颈处死后冰上取脑,分离出海马,加入预冷的RIPA裂解液和cocktail以及磷酸酶抑制剂,冰上研磨裂解,静置45 min后于4 ℃ 12 000 r/min离心5 min,取上清并进行蛋白定量,定量完毕后加入loading buffer并100 ℃煮沸5 min。进行聚丙烯酰胺凝胶电泳,80 V下10 min,130 V,50 min。电泳结束后转膜至PVDF膜上,恒压15 V,1 h。5% BSA室温封闭2 h,加入一抗,4 ℃水平摇床孵育过夜。洗膜3次,每次10 min。加入5% BSA-TBST稀释的二抗,室温孵育40 min。洗膜,显影。
1.9 统计学方法所有数据采用均数±标准差表示,运用统计学软件SPSS 17.0对所有数据进行统计学分析,进行正态性检验和方差齐性分析,组间比较采用单因素方差分析(one-way ANOVA),两两比较运用Tukey检验,析因设计采用双因素方差分析。检验水准设为α=0.05。
2 结果 2.1 侧脑室注射LPS对小鼠自发活动的影响为避免小鼠自发活动可能对水迷宫测试产生影响,我们测定了小鼠侧脑室注射LPS 24 h以后的自发活动(图 1),各组小鼠穿台次数和站立次数均没有统计学差异(P > 0.05)。
|
图 1 各组小鼠开场实验的结果 Figure 1 Results of open field test in different groups. Neither LPS nor CHO changed the times of standing (A) or times of standing (B) 24 h after LPS administration. |
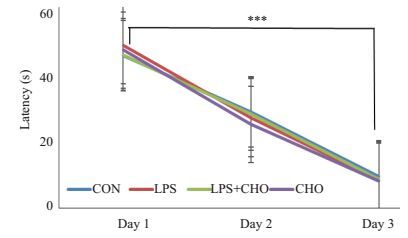
图 2表示各组小鼠水迷宫获得性训练阶段的认知功能。重复测量双因素方差分析显示动物的登台潜伏期随着训练时间和训练次数的增加而缩短(Ftime=452.1,P < 0.0001),训练第2天结束所有小鼠都能准确找到水下平台,各组小鼠在在同1 d训练的登台潜伏期差别均没有统计学意义(Fgroup=0.3161,P=0.3187),时间和分组之间没有交互作用(Finteraction=0.5304,P=0.7308)。
|
图 2 各组小鼠Morris水迷宫中定位航行实验结果 Figure 2 Place navigation in Morris water maze test. ***P < 0.05 vs training session 3 on day 3. |
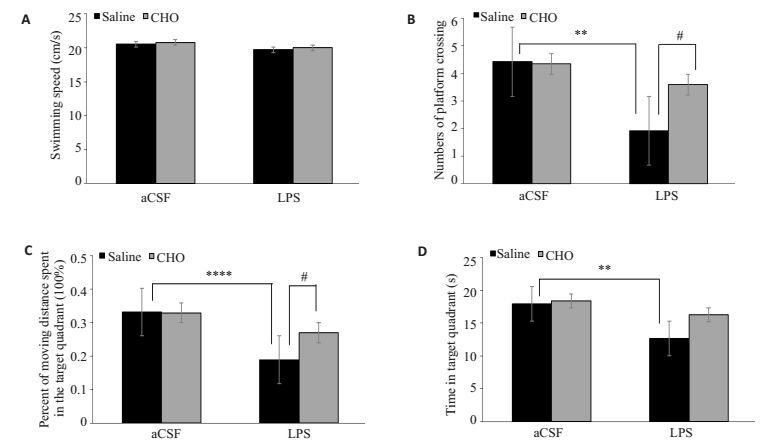
LPS暴露24 h后空间探索实验数据显示游泳速度不受LPS、CHO或者LPS×CHO交互作用的影响;LPS组小鼠穿越平台次数与对照组相比明显减少,而胆碱能够逆转LPS所致的认知障碍(Fcho=3.701,Flps=15.59,Finteration=4.521,P < 0.05);同样LPS组小鼠在目标象限停留时间和目标象限移动距离百分比均减少,该效应可被胆碱完全或部分逆转(Fcho=3.998,Flps=2.728,Finteration= 2.728,P < 0.05)(Fcho=3.410,Flps=24.32,Finteration=4.201,P < 0.05,图 3)。
|
图 3 各组小鼠Morris水迷宫空间探索实验测试结果 Figure 3 Results of spatial exploration test in Morris water maze. A: Swimming speed; B: Platform crossings; C: Percent of moving distance spent in the target quadrant; D: Percent of time spent in the target quadrant. Data are presented as Mean±SE (n=12). **P < 0.01, ****P < 0.0001; #P < 0.005. |
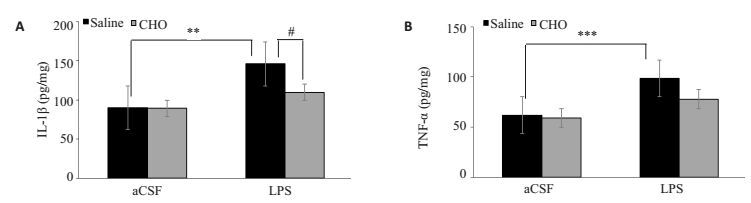
炎症因子可影响海马长时程增强效应、突触可塑性等,从而使认知功能受损。图 4A海马IL-1β炎症因子分析表明LPS(Flps=18.96,P < 0.05)占据主要效应,进一步Tukey检验表明LPS明显升高海马IL-1β水平(P < 0.01),该炎症效应可被胆碱缓解(P < 0.05)。图 4B TNF-α分析表明,表明侧脑室注射LPS会导致海马TNF-α的聚集,该效应由LPS(P < 0.0001)和LPS×CHO(P=0.0436)产生,进一步Tukey检验显示LPS显著升高TNF-α(P < 0.001),而胆碱预处理可以部分逆转这种炎症反应。
|
图 4 各组小鼠海马脑组中促炎因子水平 Figure 4 Levels of IL-1β (A) and TNF-α (B) in the hippocampus of the mice (Mean±SE, n=6). **P < 0.01, ***P < 0.001; #P < 0.05. |
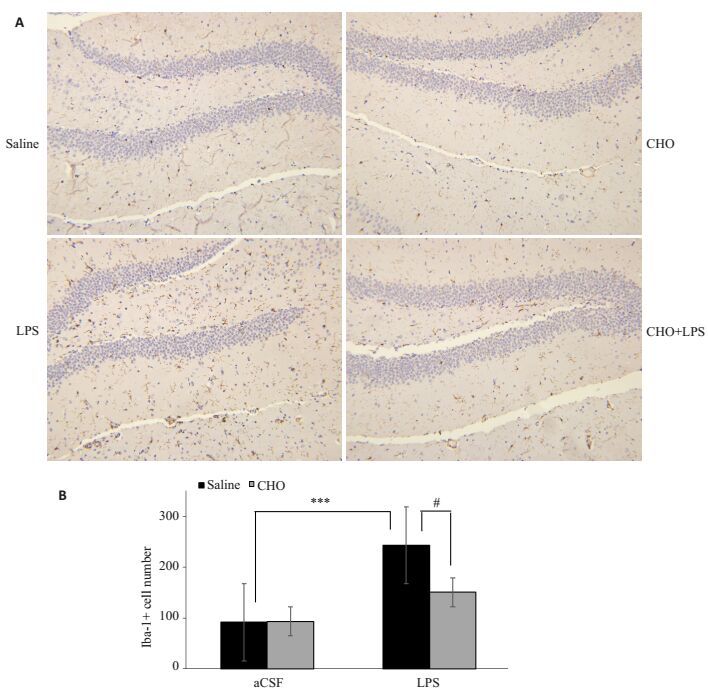
Iba-1蛋白是CNS系统小胶质细胞表面标记的离子钙接头蛋白,在小胶质细胞和巨噬细胞中特异性表达,正常生理情况下可低度表达,过度表达可以间接反应中枢炎症,可作为CNS炎症敏感的标记物。图 5显示在200倍光学显微镜下LPS侧脑室注射24 h后会导致海马齿状回Iba1标记的小胶质细胞数量显著上升(Flps=28,P < 0.0001),而胆碱可以减轻该小胶质细胞的过度激活(Fcho=5.281,P < 0.05)。
|
图 5 各组小鼠海马中小胶质细胞的激活情况 Figure 5 Microglial activation in the hippocampus of the mice. A: Representative images of iba1-labeled activated microglia in the hippocampal dentate gyrus (Original magnification: × 200); B: Number of hippocampal iba1-positive cells on postoperative day 1 (Mean±SE, n=5 or 6). ***P < 0.001; #P < 0.05. |
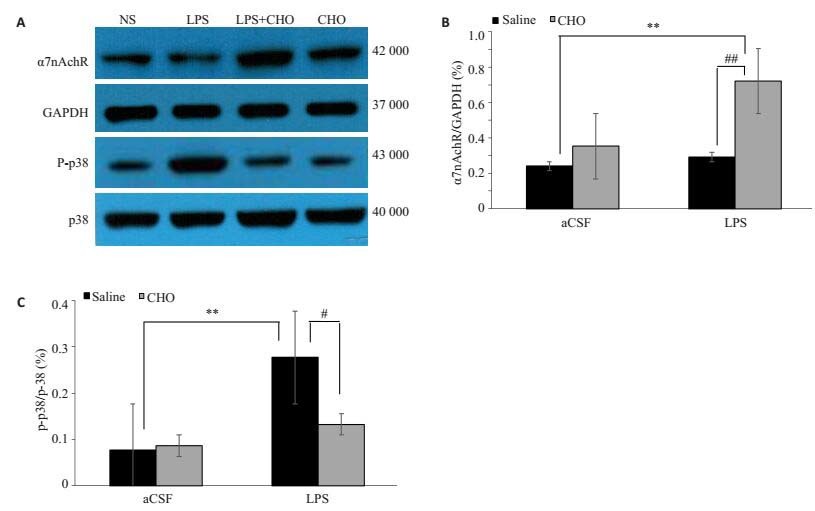
胆碱是选择性α7nAchR激动剂,图 6A结果表明其可特异性激活海马病理状态下的α7nAchR并上调其表达(Fcho=19.59,P=0.002,图 6A,C)。
|
图 6 各组小鼠海马中α7nAchR和p-p38蛋白表达情况 Figure 6 Expression of α7nAchR and p-p38 proteins in the hippocampus of the mice. The expression levels of α7nAchR and p-p38/p38 proteins were normalized to that of GAPDH (internal control). **P < 0.01, #P < 0.05, ##P < 0.01. |
图 6B检测表明LPS暴露后p38蛋白磷酸化水平明显增加(Flps=24.6,P < 0.05,图 6B,D),而胆碱能够降低p38蛋白的磷酸化(Fcho=7.182,P < 0.05,图 6B,D)。
3 讨论胆碱是人类日常必需的营养物质,作为α7nAchR选择性激动剂,诸多研究证实胆碱除对脑缺血有保护作用外,在各种神经精神疾病模型包括自闭症[9]、唐氏综合症[10]、癫痫[11]、酒精谱系疾病[12]等,都有证明具有神经保护效应。近年关于胆碱对炎症及认知功能影响的研究日益增多,有研究提示其可通过胆碱能抗炎通路对脓毒血症以及多种炎症模型产生抑炎效应。本研究结果表明胆碱可改善LPS所致CNS炎症反应及认知功能障碍,此机制可能涉及α7nAchR信号通路和p38MAPK信号通路。
Morris水迷宫测试成绩可反映海马依赖性学习记忆能力,因此我们选用该实验检测小鼠参考记忆,同时选定LPS 2 μg脑室内微注射,该剂量能够更有效的引起小鼠术后24 h的记忆功能损害[13]。本实验结果表明小鼠侧脑室注射LPS 24 h后Morris水迷宫的空间学习记忆能力下降,登台潜伏期显著延长、目标象限停留时间和穿台次数比其他组少。由于胆碱40、60、80 mg/kg均能降低LPS诱导的小鼠脓毒血症24 h后的死亡率以及炎症因子的产生[14],因此在预实验中,我们探索这几个剂量梯度对LPS所致小鼠认知功能损伤的保护效应,我们发现胆碱40 mg/kg干预组小鼠的水迷宫测试成绩优于60 mg/kg及80 mg/kg,能够更有效地改善LPS所致小鼠的认知损伤,这一剂量比Pan等报告的每日单次给药的最佳剂量60 mg/kg小,其研究结果显示此剂量抵抗LPS所致小鼠脓毒血症的效果最好[14],原因可能是胆碱的血浆半衰期比较短,在70 min左右,每天连续给药3次能更好地维持血浆最佳有效浓度;另外模型不一样药物干预的效果可能也会不一样。
神经退行性疾病伴常有慢性CNS炎症,而CNS炎症可促使免疫系统胶质细胞激活[15],炎症因子、氧自由基和兴奋性神经毒素释放并损伤突触可塑性,最终使记忆功能受损。脑室或腹腔注射LPS可产生CNS炎症,其主要作用于TLR4/CD14炎症复合体并介导其下游IKKα/β、NFκB炎症信号通路的激活,促使神经胶质细胞和COX-2、TNF-α、iNOS、IL-1β等促炎介质活化,其后续能够促进阿尔兹海默病特征性病理标记物Aβ、APP、BACE-1、p-Tau的聚积,使PSD-95、SNAP-25、syntaxin-1等突触功能蛋白表达降低从而损伤突触前后功能,可上调PARP-1、caspase-3等凋亡蛋白的表达从而促使凋亡进程,亦可加速氧化应激进程[13, 16],也可降低脑源性神经营养因子表达,最终损害认知功能[17]。LPS暴露12 h以内可引起自发活动和社交行为的改变,而24 h后抑郁样、认知功能受损等病态行为更为明显,体重下降显著并伴随一系列病理性变化。小胶质细胞激活后促炎因子在6 h以内达峰,24 h后回归基线[13, 18-19],因此抑制小胶质细胞的激活和减少神经毒性细胞因子就成为治疗神经退行性变的主要策略。本研究结果表明侧脑室注射LPS 24 h后能够显著增强海马齿状回Iba-1蛋白免疫活性,而胆碱预处理后明显抑制海马小胶质细胞的异常激活,使Iba-1免疫活性保持在基线水平。由于小胶质细胞激活后主要释放TNF-α、IL-1β等炎性因子,为了进一步确证胆碱的抗炎特性,本研究结果发现胆碱预处理能够抑制TNF-α、IL-1β的上调,同样Guseva等[20]研究也提示胆碱可通过α7nAchR信号通路抑制小鼠胫骨骨折术后血清TNF-α、IL-1β、HMGB-1的升高,基于胆碱对LPS所致小鼠CNS反应也有类似的抗炎效应,我们继续探索其抗炎特性与7nAchR信号通路的关系。
α7nAchR是迷走神经胆碱能抗炎通路的一个重要组成部分,胆碱作为天然的α7nAchR激动剂,能够通过α 7受体抑制LPS诱导的RAW细胞NF-κB的激活,降低内毒素小鼠全身性TNF水平,而敲除该受体之后胆碱无法产生抗炎效应[21];有相关研究报道胆碱在狗内毒素血症模型上能够降低肝脏合成炎症相关的对氧磷酯酶及丁酰胆碱酯酶[22];也有研究提示胆碱可降低肝肺巨噬细胞内钙离子浓度以及TNF-α水平,从而降低内毒素休克大鼠的死亡率[23];有研究者还发现胆碱可降低内毒素急性反应期基质金属蛋白酶表达并维持较高浓度的免疫球蛋白IgG、IgM水平[24]。本研究结果表明在生理盐水组、LPS组以及胆碱对照组中α7nAchR表达没有明显升高,而在胆碱干预组中有显著的提高,表明胆碱在LPS诱导的病理状态下可能会通过激活并上调α 7nAchR表达而发挥相应的抗炎效应,而在正常个体中无论是否使用胆碱,该受体表达均无明显差异,与Li等通过基因构建技术上调α7nAchR表达产生更加强烈的抑炎效应的结论相类似[25]。
丝裂原活化蛋白激酶(MAPK)是一类苏氨酸/丝氨酸特异性激酶,其中的p38MAPK信号转导通路参与了细胞发育及细胞功能同步等多种生理过程,在急慢性炎症过程中,激活该通路可促使白细胞激活并到达炎症靶点参与炎症反应[26]。有研究表明α7nAchR激动剂乙酰胆碱和烟碱可通过降低p38MAPK的磷酸化来抑制LPS诱导的小胶质细胞TNF-α的释放,敲除α 7nAchR基因或运用该受体选择性抑制剂α金环蛇毒素后,该抗炎效应可被阻断[27-28],基于此,我们进一步探索胆碱的抗炎效应与p38MAPK信号通路的关系,本实验结果表明LPS能够显著增加小鼠海马区p38蛋白的磷酸化水平,而胆碱能够抑制该磷酸化效应。同样Jiao等[29]发现烟碱在肥胖引起的慢性炎症中可通过激活α 7nAchR来抑制p38激酶和NF-κB的激活;Li等[30]也发现乙酰胆碱可通过降低p38MAPK以及C-JNK的磷酸化来降低心肌细胞缺氧损伤引起的TNF-α的升高,胆碱与烟碱、乙酰胆碱共同作为α7nAchR激动剂,其可能类似地通过抑制p38MAPK信号通路来产生抗炎效应。
总之,胆碱能够改善LPS侧脑室注射引起的小鼠CNS炎症反应及认知功能障碍,其保护机制可能是通过激活α7nAchR来降低p38MAPK磷酸化水平,从而抑制海马小胶质细胞的激活以及促炎因子的产生。胆碱作为神经营养类药物之一,可为神经退行性疾病诊疗策略提供新的治疗方法。
| [1] | Wang P, Cao J, Liu N, et al. Protective effects of edaravone in adult rats with surgery and lipopolysaccharide Administration-Induced cognitive function impairment[J]. PLoS One, 2016, 11(4): e0153708. DOI: 10.1371/journal.pone.0153708. |
| [2] | Lykhmus O, Mishra N, Koval L, et al. Molecular mechanisms regulating LPS-Induced inflammation in the brain[J]. Front Mol Neurosci, 2016, 9: 19. |
| [3] | 刘犇, 陈清, 郭江, 等. 白藜芦醇对脂多糖诱导的星型胶质细胞炎症损伤的保护作用[J]. 南方医科大学学报, 2011, 31(12): 2052-6. |
| [4] | Bales KR, Du Y, Holtzman D, et al. Neuroinflammation and alzheimer's disease:critical roles for cytokine/abeta-induced glial activation, NF-kappaB, and apolipoprotein E[J]. Neurobiol Aging, 2000, 21(3): 427-32. DOI: 10.1016/S0197-4580(00)00143-3. |
| [5] | Zhang H, Ma L, Yin YL, et al. Over-expression of TSPO in the hippocampal CA1 area alleviates cognitive dysfunction caused by lipopolysaccharide in mice[J]. Brain Res, 2016, 1646: 402-9. DOI: 10.1016/j.brainres.2016.06.001. |
| [6] | Ali MR, Abo-Youssef AM, Messiha BA, et al. Tempol and perindopril protect against lipopolysaccharide-induced cognition impairment and amyloidogenesis by modulating brain-derived neurotropic factor, neuroinflammation and oxido-nitrosative stress[J]. Naunyn Schmiedebergs Arch Pharmacol, 2016, 389(6): 637-56. DOI: 10.1007/s00210-016-1234-6. |
| [7] | Jin X, Wang RH, Wang H, et al. Brain protection against ischemic stroke using choline as a new molecular bypass treatment[J]. Acta Pharmacol Sin, 2015, 36(12): 1416-25. DOI: 10.1038/aps.2015.104. |
| [8] | Guseva MV, Hopkins DM, Scheff SW, et al. Dietary choline supplementation improves behavioral, histological, and neurochemical outcomes in a rat model of traumatic brain injury[J]. J Neurotrauma, 2008, 25(8): 975-83. DOI: 10.1089/neu.2008.0516. |
| [9] | Langley EA, Krykbaeva M, Blusztajn JK, et al. High maternal choline consumption during pregnancy and nursing alleviates deficits in social interaction and improves anxiety-like behaviors in the BTBR T+Itpr3tf/J mouse model of autism[J]. Behav Brain Res, 2015, 278: 210-20. DOI: 10.1016/j.bbr.2014.09.043. |
| [10] | Kelley CM, Ash JA, Powers BE, et al. Effects of maternal choline supplementation on the septohippocampal cholinergic system in the Ts65Dn mouse model of down syndrome[J]. Curr Alzheimer Res, 2015, 13(1): 84-96. DOI: 10.2174/1567205012666150921100515. |
| [11] | Wong-Goodrich SJ, Glenn MJ, Mellott TJ, et al. Water maze experience and prenatal choline supplementation differentially promote long-term hippocampal recovery from seizures in adulthood[J]. Hippocampus, 2011, 21(6): 584-608. DOI: 10.1002/hipo.v21.6. |
| [12] | Kable J, Coles C, Chambers C, et al. The impact of micronutrient supplementation in alcohol-exposed pregnancies on information processing skills in Ukrainian infants[J]. Alcohol, 2013, 48(1): 22. |
| [13] | Zhang XY, Cao JB, Zhang LM, et al. Deferoxamine attenuates lipopolysaccharide-induced neuroinflammation and memory impairment in mice[J]. J Neuroinflammation, 2015, 12: 20. DOI: 10.1186/s12974-015-0238-3. |
| [14] | Pan ZY, Wang H. Synergistic interaction between choline and aspirin against acute inflammation induced by carrageenan and lipopolysaccharide[J]. Int Immunopharmacol, 2014, 20(1): 229-37. DOI: 10.1016/j.intimp.2014.03.004. |
| [15] | Conde JR, Streit WJ. Microglia in the aging brain[J]. J Neuropathol Exp Neurol, 2006, 65(3): 199-203. DOI: 10.1097/01.jnen.0000202887.22082.63. |
| [16] | Badshah H, Ali T, Kim MO. Osmotin attenuates LPS-induced neuroinflammation and memory impairments via the TLR4/NFκB signaling pathway[J]. Sci Rep, 2016, 6: 24493. DOI: 10.1038/srep24493. |
| [17] | Hou Y, Xie G, Liu X, et al. Minocycline protects against lipopolysaccharide-induced cognitive impairment in mice[J]. Psychopharmacology(Berl), 2016, 233(5): 905-16. DOI: 10.1007/s00213-015-4169-6. |
| [18] | Norden DM, Trojanowski PJ, Villanueva E, et al. Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge[J]. Glia, 2016, 64(2): 300-16. DOI: 10.1002/glia.22930. |
| [19] | Lawson MA, Mccusker RH, Kelley KW. Interleukin-1 beta converting enzyme is necessary for development of depression-like behavior following intracerebroventricular administration of lipopolysaccharide to mice[J]. J Neuroinflammation, 2013, 10: 54. |
| [20] | Terrando N, Eriksson LI, Ryu JK, et al. Resolving postoperative neuroinflammation and cognitive decline[J]. Ann Neurol, 2011, 70(6): 986-95. DOI: 10.1002/ana.22664. |
| [21] | Parrish WR, Rosas-Ballina M, Gallowitsch-Puerta M, et al. Modula-tion of TNF release by choline requires alpha7 subunit nicotinic ace-tylcholine receptor-mediated signaling[J]. Mol Med, 2008, 14(9/ 10): 567-74. |
| [22] | Tvarijonaviciute A, Kocaturk M, Cansev M, et al. Serum butyryl-cholinesterase and paraoxonase 1 in a canine model of endotox-emia:effects of choline administration[J]. Res Vet Sci, 2012, 93(2): 668-74. DOI: 10.1016/j.rvsc.2011.09.010. |
| [23] | Rivera CA, Wheeler MD, Enomoto N, et al. A choline-rich diet improves survival in a rat model of endotoxin shock[J]. Am J Physiol, 1998, 275(4Pt 1): G862-7. |
| [24] | Kocaturk M, Eralp-Inan O, Tvarijonaviciute A, et al. Effects of choline treatment in concentrations of serum matrix metalloproteinases(MMPs), MMP tissue inhibitors(TIMPs)and immunoglobulins in an experimental model of canine sepsis[J]. Vet Immunol Immunopathol, 2016, 180: 9-14. DOI: 10.1016/j.vetimm.2016.08.011. |
| [25] | Li DJ, Tang Q, Shen FM, et al. Overexpressed alpha7 nicotinic acetylcholine receptor inhibited proinflammatory cytokine release in NIH3T3 cells[J]. J Biosci Bioeng, 2009, 108(2): 85-91. DOI: 10.1016/j.jbiosc.2009.03.004. |
| [26] | Herlaar E, Brown Z. p38 MAPK signalling cascades in inflammatory disease[J]. Mol Med Today, 1999, 5(10): 439-47. DOI: 10.1016/S1357-4310(99)01544-0. |
| [27] | Shytle RD, Mori T, Townsend K, et al. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors[J]. J Neurochem, 2004, 89(2): 337-43. DOI: 10.1046/j.1471-4159.2004.02347.x. |
| [28] | Yi L, Luo JF, Xie BB, et al. α7 nicotinic acetylcholine receptor is a novel mediator of sinomenine anti-Inflammation effect in macrophages stimulated by lipopolysaccharide[J]. Shock, 2015, 44(2): 188-95. DOI: 10.1097/SHK.0000000000000389. |
| [29] | Jiao ZY, Wu J, Liu C, et al. Nicotinic α7 receptor inhibits the acylation stimulating protein-induced production of monocyte chemoattractant protein-1 and keratinocyte-derived chemokine in adipocytes by modulating the p38 kinase and nuclear factor-κB signaling pathways[J]. Mol Med Rep, 2016, 14(4): 2959-66. |
| [30] | Li DL, Liu JJ, Liu BH, et al. Acetylcholine inhibits hypoxia-induced tumor necrosis factor-α production via regulation of MAPKs phosphorylation in cardiomyocytes[J]. J Cell Physiol, 2011, 226(4): 1052-9. DOI: 10.1002/jcp.22424. |
 2017, Vol. 37
2017, Vol. 37

