2. Department of Blood Transfusion, General Hospital of PLA, Beijing 100853, China
2. 解放军总医院输血科实验室,北京 100853
Thin endometrium can cause poor uterine receptivity to affect embryo implantation and ultimately reduce the pregnancy rate[1]. In spite of the various approaches that have been attempted, so far none of them seemed to produce a definite therapeutic effect in treatment of thin endometrium, which still remains a clinical challenge[2]. Currently the treatments of thin endometrium include hormonal manipulation by estrogen and gonadotropin-releasing hormone agonist; vasoactive agents such as aspirin, vitamin E, pentoxifylline, L-arginine or sildenafil; intrauterine infusion of such growth factors as granulocyte-colony stimulating factor (G-CSF); and the recent application of regenerative medicine[3].
Adipose-derived stem cells (ADSCs) are capable of differentiation into multiple cell lineages, including osteoblasts, cartilage cells, adipocytes, myocytes, vascular endothelial cells, and neurons[4]. ADSCs are characterized by a low immunogenicity and stable proliferation and can be ideal seed cells for autologous cell repair and regeneration[5]. Stem cell therapy, however, is also associated with potentials risks when the stem cells are directly used for treatment, as the strong proliferation and differentiation capacities of the cells may promote tumor growth and even give rise to the formation of tumors[6, 7].
The study by Andrade et al[8] showed that gamma irradiation of human bone marrow-derived mesenchymal stem cells (hBMSCs) produced suppression of cell proliferation; exposure of the cells to a dose above 25 Gy caused cell proliferation arrest but retained the immunosuppressive potential and inhibited the clonogenic capacity of the cells. In this study, we aimed to test the proliferative capacity of ADSCs following gamma irradiation and determine the optimal radiation dose. We evaluated the effects of gamma-irradiated ADSCs in the treatment of thin endometrium in a rat model by observing the changes in the morphology and thickness of the endometrium.
MATERIALS AND METHODS AnimalsForty female adult Sprague-Dawley (SD) rats weighing 220-280 g were maintained in separated cages at a controlled temperature of 24 ℃ with free access to food and water. A 14 h/10 h light/dark cycle was maintained. The study was reviewed and approved by the Animal Experimental Center of General Hospital of PLA. All the procedures of animal manipulation were approved by the Institutional Animal Care and Use Committee.
Establishment of thin endometrium modelRat models of thin endometrium were established acco-rding to the protocols described in a previous study[3]. Briefly, the rats were anesthetized with an intra-peritoneal injection of 10% chloral hydrate (0.4 g/kg)[9], the uterus was exposed, each uterine horn was clipped with a vascular clip, and 95% alcohol (0.5 mL) was injected into the uterine horn using a 1 mL syringe with a 16-gauge needle. After the modeling, the rats were kept in incubation chambers with a controlled temperature and humidity until they awoke.
Acquisition, identification, and labeling of ADSCsADSCs were isolated following the protocols previously reported[10]. Briefly, the subcutaneous adipose tissue was acquired from the inguinal region of the rats and washed thoroughly with sterile phosphate-buffered saline (PBS) to remove the contaminating debris and red blood cells. The tissue was then cut into small pieces with scissors and digested with 0.1% type Ⅰ collagenase (Sigma, US) in serum-free medium at 37 ℃ with gentle agitation. After digestion for 30 to 60 min, the enzymes were inactivated with an equal volume of α-MEM (Gibco, US) supplemented with 10% fetal bovine serum (FBS, Gibco), and the samples were filtered through a 200-μm mesh filter to remove the debris followed by centrifugation at 600 g for 5 min. The cellular pellets were cultured in α-MEM/10% FBS supplemented with 50 U/mL penicillin and 50 μg/mL streptomycin in a humidified incubator at 37 ℃ with 5% carbon dioxide. The third passage of ADSCs was used for the transplantation experiments.
The expression of surface markers on the ADSCs was analyzed with flow cytometry using fluorescein isothiocyanate (FITC)-conjugated mouse monoclonal antibodies (mAbs) against CD45 and CD34, and phycoerythrin (PE)-conjugated mouse mAbs against CD29 and CD90 (Biolegend, US). For flow cytometric analysis, the adherent cells were detached by treatment with 0.25% trypsin-EDTA, neutralized with FBS-containing culture medium, and disaggregated into single cells by pipetting. The cells were incubated with mAbs for 30 min at 4 ℃, washed twice with PBS, resuspended in 0.5 mL PBS, and immediately analyzed with an FACS Calibur flow cytometer (Becton Dickinson, US). A minimum of 2×105 cells were used for each sample, and cell Quest software was used for data analysis.
To confirm the multipotency of the ADSCs, the cells were tested for osteogenic and adipogenic differentiation with Alizarin Red staining and Oil Red O staining, respectively[11]. For induction of osteogenic differentiation, the cells were seeded in 6-well plate at a density of 2 × 103 cells/cm2. After 24 h, the medium was replaced by osteogenic differentiation medium, and the cells were induced for 3 weeks followed by examination with Alizarin Red staining. For osteogenic differentiation, the cells were seeded at a density of 1 × 104/cm2 and after reaching confluency, the cells were incubated in adipogenic differentiation medium for 2 weeks and examined with Oil Red O staining. During the induction, the differentiation medium was changed every 3 days.
Irradiation of ADSCsADSCs were irradiated according to the protocols reported previously[8]. ADSC suspension in tubes were irradiated using the Gamma-cell 3000 Elan device (Best Theratronics, Ottawa, ON, Canada) at the doses of 5 and 10 Gy, and the non-irradiated control cells were kept at room temperature. Both the irradiated and non-irradiated ADSCs were washed for further assays. For transplantation experiment, the cells were irradiated within the flasks, washed and maintained in culture until the day of harvest.
Colony forming unit (CFU) assayUpon reaching 80-90% confluency, ADSCs were harvested and irradiated as described above, washed, counted and plated in 6-well plates at the density of 50 cells/cm2 (500 cells/well) with 2 mL of α-MEM (Gibco, US). The plates were incubated at 37 ℃ in the presence of 5% CO2 for 14 days to observe colony formation. After that, the ADSCs were stained with 0.5% crystal violet (Sigma-Aldrich) for 5 min at room temperature and washed. For better colony visualization, 2 mL of PBS was added to each well and the colonies were counted.
Animal groupingThe rats were kept under standardized laboratory conditions in an air conditioned room with free access to food and water. Twenty-four rats were randomly assigned to 4 groups (n=6), including 3 experimental groups and one control group. At 6-8 h after modeling, the rats in the experimental groups received intrauterine injection of non-irradiated ADSCs (group Ⅰ), 5 Gy irradiated ADSCs (group Ⅱ), or 10 Gy irradiated ADSCs (group Ⅲ). A preliminary experiment was done to characterize the extent of endometrial cell injury when ADSCs were infused. In each rat, 1 mL α-MEM containing 4 × 107 ADSCs/mL was injected, and the control rats received injection of an equivalent volume of PBS. In the third estrus phase (determined by observing the vaginal smear) after injection of ADSCs, the rats were sacrificed by intraperitoneal injection of an overdose of 10% chloral hydrate (1.0 g/kg). The uteri were excised, sectioned and preserved in formalin and/ or liquid nitrogen for HE staining.
Statistical analysisThe measurement data are presented as Mean ± SD. Comparisons among multiple groups were performed with one-way analysis of variance (ANOVA) using SPSS version 16.0 (IBM). A P value less than 0.05 was considered to indicate a statistically significant difference.
RESULTS Culture and characterization of ADSCsThe isolated cells were cultured in tissue-culture dishes. After 7 days in culture, the cells presented with a spindle-shaped morphology and symmetric colonies were formed (Fig. 1A). Surface marker analysis of the cells in the third passage with flow cytometry showed that most of the adherent cells ( > 95%) expressed CD29 and CD90 (Figure 1B). The majority of the adherent cells were negative for CD34 and CD45 (Fig. 1B). The cell differentiation tests showed that the isolated ADSCs were multipotent and capable of differentiating into osteoblasts and adipocytes (Fig. 1C).
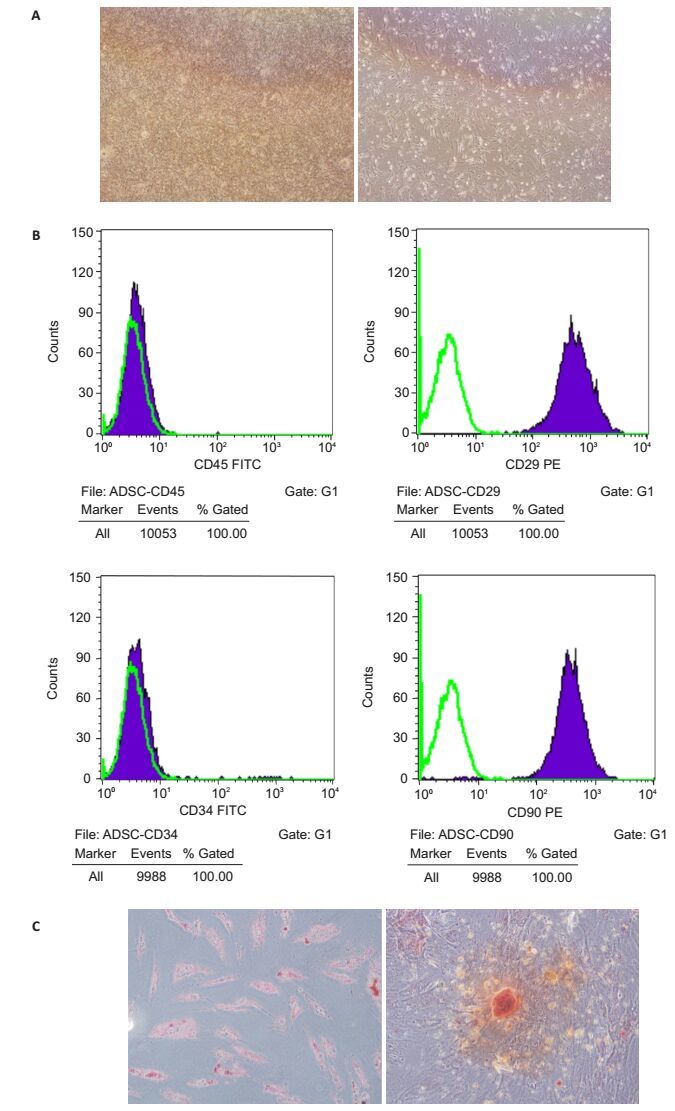
|
Figure 1 Characterization of the isolated ADSCs in the 4-6 passages.A: ADSCs showing a typical fibroblastic morphology(Original magnification: × 40); B: Flow cytometry for detecting the surface markers (CD45, CD29, CD34, and CD90); C: Differentiation potential of the ADSCs into osteoblasts (left, Alizarin Red staining) and adipocytes (right, Oil Red O staining) assessed by the presence of calcium precipitates and lipid droplets (×40). |
Ionizing radiation was previously shown to successfully inhibit proliferation of lymphocytes and reduce mesenchymal stem cell (MSC) proliferation[8]. However, the sensitivity of ADSCs to irradiation in terms of colony-forming potential has not been shown. We exposed the ADSCs in the 4th passage to irradiation at 5 and 10 Gy. After cultivation for 2 weeks, the non-irradiated ADSCs yielded 30.3 ± 1.9 colonies/well; the cells exposed to 5 Gy irradiation formed less than 10 colonies, and a 10 Gy exposure totally abrogated colony formation by the cells. The culture flasks containing non-irradiated control ADSCs exhibited massive colony overgrowth after 16 days.
Irradiated ADSC transplantation promotes endometrial cell regenerationThe endometrial thickness, superficial epithelia of the endometrium, and the number of endometrial glands differed significantly between the rats with ADSCs transplantation and the rats in the control group. No significant difference was noted in the therapeutic effect between group Ⅰ and group Ⅱ. Histological evaluation of the uterine in the experimental groups showed intact structure of the endometrial layer characterized by an increased endometrial thickness and increased numbers of endometrial glands and capillaries. The uterine horn in the control group was completely destroyed with extensive necrosis, and coagulative necrosis was detected in the endometrium layer and the myometrium layer; some rats even showed total loss of normal endometrial tissues.
The endometrial thickness was significantly greater in experimental groups Ⅰ and Ⅱ than in the control group (P < 0.01), but there was no significant difference between the former two groups (P > 0.05). No significant difference was found between group Ⅲ and the control group (Fig. 2).
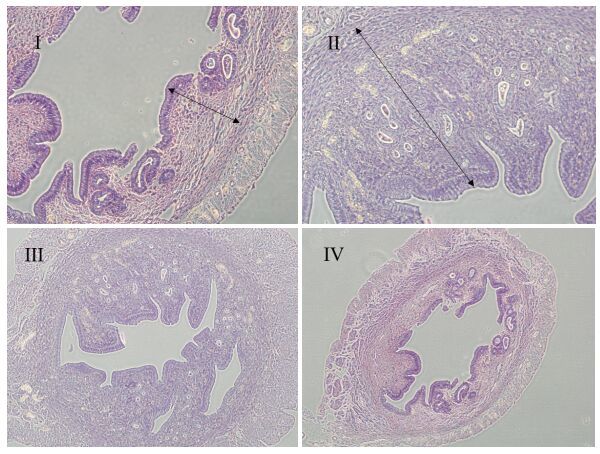
|
Figure 2 Pathological examination of the endometrium in rats with thin endometrium treated with intrauterine injection of non-irradiated ADSCs (Ⅰ), 5 Gy irradiated ADSCs (Ⅱ), 10 Gy irradiated ADSCs (Ⅲ), or PBS (Ⅳ) (HE staining, × 40). The endometrial lining was thicker in groups Ⅰ and group Ⅱ than in group Ⅳ, and was similar between groups Ⅰ and Ⅱ. |
In view of the patient safety, the clinical applications of MSCs, even in compassionate use, should consider the potential risks such as secondary engraftment, which might lead to ectopic tissue formation or tumor formation caused by malignant transformation[8, 12, 13].
Thin endometrium is a difficult problem in assisted reproductive technique (ART) with an unknown prevalence. Practitioners are striving to solve this problem and improve the pregnancy chances of infertile patients with thin endometrium[14]. Researchers have tested different regimens for treatment of thin endo-metrium, including extended estrogen administration[15], low-dose aspirin[16], vaginal sildenafil citrate[17], the combination of pentoxifylline and tocopherol[18, 19], and gonadotropin-releasing hormone agonist[20]. Some therapies improved the endometrial thickness, and some did not. So far, none of these treatments is widely accepted for management of thin endometrium. New treatment approaches have also been tested for thin endometrium resistant to conventional therapy, such as G-CSF and regenerative medicine. Several routes have been reported for the delivery of stem cells for endometrium repair, including intravenous and in situ injections[21, 22]. However, currently no studies have been reported to test the effect of irradiated ADSC transplantation in the treatment of thin endometrium.
We previously tested BMSC transplantation for treatment of thin endometrium in rats and observed a positive effect[23]. In this study, we transplanted ADSCs in the rat models after irradiation of the cells to improve the safety of stem cell therapy. We showed that the rats in the experimental groups had a thicker endometrium than those in the control group, indicating that the ADSCs exposed to 5 Gy irradiation still produced therapeutic effect on thin endometrium induced by ethanol. This seems to suggest that the effect of stem cell therapy is not completely dependent on the proliferation and differentiation of the stem cells, but might be related to the exosomes and special substances released by the stem cells to promote the regeneration of the endometrial cells. The ADSCs exposed to 5 Gy gamma irradiation showed a weakened proliferative capacity, which suggests their lowered potential of oncogenicity; but they were still capable of promoting endometrial cell regeneration.
| [1] | Lebovitz O, Orvieto R. Treating patients with'thin' endometrium-an ongoing challenge[J]. Gynecol Endocrinol, 2014, 30(6): 409-14. DOI: 10.3109/09513590.2014.906571. |
| [2] | Jimenez PT, Schon SB, Odem RR, et al. A retrospective cross-sectional study:fresh cycle endometrial thickness is a sensitive predictor of inadequate endometrial thickness in frozen embryo transfer cycles[J]. Reprod Biol Endocrinol, 2013, 11: 35. DOI: 10.1186/1477-7827-11-35. |
| [3] | Zhao J, Tian T, Zhang Q, et al. Use of granulocyte colony-stimulating factor for the treatment of thin endometrium in experimental rats[J]. PLoS One, 2013, 8(12): e82375. DOI: 10.1371/journal.pone.0082375. |
| [4] | Min Sun, Shufang Wang, Yi Li, et al. Adipose-derived stem cells improved mouse ovary function after chemotherapy-induced ovary failure[J]. Stem Cell Res Ther, 2013, 4(4): 80. DOI: 10.1186/scrt231. |
| [5] | Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells[J]. Mol Biol Cell, 2002, 13: 4279-95. DOI: 10.1091/mbc.E02-02-0105. |
| [6] | Torsvik A, Rosland GV, Svendsen A, et al. Spontaneous malignant transformation of human mesenchymal stem cells reflects cross-contamination:putting the research field on track-letter[J]. Cancer Res, 2010, 70(15): 6393-6. DOI: 10.1158/0008-5472.CAN-10-1305. |
| [7] | Amariglio N, Hirshberg A, Scheithauer BW, et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient[J]. PLoS Med, 2009, 6(2): e1000029. DOI: 10.1371/journal.pmed.1000029. |
| [8] | De Andrade AV, Riewaldt J, Wehner R, et al. Gamma irradiation preserves immunosuppressive potential and inhibits clonogenic capacity of human bone marrow-derived mesenchymal stromal cells[J]. Cell Mol Med, 2014, 18(6): 1184-93. DOI: 10.1111/jcmm.2014.18.issue-6. |
| [9] | Harvey BK, Airavaara M, Hinzman J, et al. Targeted over-expression of glutamate transporter 1(GLT-1) reduces ischemic brain injury in a rat model of stroke[J]. PLoS One, 2011, 6: e22135. DOI: 10.1371/journal.pone.0022135. |
| [10] | Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine[J]. Circ Res, 2007, 100: 1249-60. DOI: 10.1161/01.RES.0000265074.83288.09. |
| [11] | Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells[J]. Mol Biol Cell, 2002, 13: 4279-95. DOI: 10.1091/mbc.E02-02-0105. |
| [12] | Bernardo ME, Fibbe WE. Safety and efficacy of mesenchymal stromal cell therapy in autoimmune disorders[J]. Ann N Y Acad Sci, 2012, 1266: 107-17. DOI: 10.1111/nyas.2012.1266.issue-1. |
| [13] | Kramann R, Kunter U, Brandenburg VM, et al. Osteogenesis of heterotopically transplanted mesenchymal stromal cells in rat models of chronic kidney disease[J]. J Bone Miner Res, 2013, 28: 2523-34. DOI: 10.1002/jbmr.1994. |
| [14] | Yu L, Ping P, Chen X, et al. Granulocyte colony-stimulating factor administration for infertile women with thin endometrium in frozen embryo transfer program[J]. Reprod Sci, 2014, 21(3): 381-5. DOI: 10.1177/1933719113497286. |
| [15] | Groenewoud ER, Cantineau AEP, Kollen BJ, et al. What is the optimal means of preparing the endometrium in frozen-thawed embryo transfer cycles? A systematic review and meta-analysis[J]. Hum Reprod Update, 2013, 19: 458-70. DOI: 10.1093/humupd/dmt030. |
| [16] | Weckstein LN, Jacobson A, Galen D, et al. Low-dose aspirin for oocyte donation recipients with a thin endometrium:prospective, randomized study[J]. Fertil Steril, 1997, 68: 927-30. DOI: 10.1016/S0015-0282(97)00330-0. |
| [17] | Sher G, Fisch JD. Effect of vaginal sildenafil on the outcome of in vitro fertilization(IVF)after multiple IVF failures attributed to poor endometrial development[J]. Fertil Steril, 2002, 78: 1073-6. DOI: 10.1016/S0015-0282(02)03375-7. |
| [18] | Letur-K nirsch H, Guis F, Delanian S. Uterine restoration by radiation sequelae regression with combined pentoxifylline-tocopherol:a phase Ⅱ study[J]. Fertil Steril, 2002, 77: 1219-26. DOI: 10.1016/S0015-0282(02)03120-5. |
| [19] | Acharya S, Yasmin E, Balen AH. The use of a combination of pentoxifylline and tocopherol in women with a thin endometrium undergoing assisted conception therapies:a report of 20 cases[J]. Hum Fertil(Camb), 2009, 12: 198-203. DOI: 10.3109/14647270903377178. |
| [20] | Tesarik J, Hazout A, Mendoza C, Tesarik R, et al. Beneficial effect of luteal-phase GnRH agonist administration on embryo implantation after ICSI in both GnRH agonist and antagonist-treated ovarian stimulation cycles[J]. Hum Reprod, 2006, 21: 2572-9. DOI: 10.1093/humrep/del173. |
| [21] | Nagori CB, Panchal SY, Patel H. Endometrial regeneration using autologous adult stem cells followed by conception by in vitro fertilization in a patient of severe Asherman's syndrome[J]. J Hum Reprod Sci, 2011, 4: 43-8. DOI: 10.4103/0974-1208.82360. |
| [22] | Gargett CE, Healy DL. Generating receptive endometrium in Asherman's syndrome[J]. J Hum Reprod Sci, 2011, 4: 49-52. |
| [23] | Jing Z, Qiong Z, Yonggang W, et al. Rat bone marrow mesenchymal stem cells improve regeneration of thin endometrium in rat[J]. Fertil Steril, 2014, 101: 587-94. DOI: 10.1016/j.fertnstert.2013.10.053. |
 2017, Vol. 37
2017, Vol. 37
