2. 蚌埠医学院,组织移植安徽省重点实验室,安徽 蚌埠 233030;
3. 第一附属医院检验科,安徽 蚌埠 233004
2. Anhui Key Laboratory of Tissue Transplantation, Bengbu 233030, China;
3. Clinical Laboratory, First Affiliated Hospital of Bengbu Medical College, Bengbu 233004, China
少突胶质细胞(OLs)是中枢神经系统的髓鞘形成细胞[1-2] ,少突胶质前体细胞(OPCs)是OLs的前体细胞[1-3] ,OPCs表达特异性标志A2B5、NG2 和PDGFRa,体外培养的OPCs在无血清条件下分化为OLs,而在血清存在条件下分化为II型星型胶质细胞[4-6] 。众所周知,在体外培养中,大鼠神经母细胞瘤细胞株B104的条件培养液(B104CM)促进OPCs的增殖,被广泛用于体外OPCs的培养与扩增[7-8] 。研究证明B104CM通过分泌的PDGF-AA和碱性纤维细胞生长因子(bFGF)[9] 激活细胞外调节蛋白激酶[10] 和JNK[11] 信号途径诱导OPCs的增殖。
雪旺细胞是周围神经系统的髓鞘形成细胞[12-14] 。SCs在体内外均促进OPCs的增殖[15] ,并证明SCs也是通过分泌PDGF-AA 和bFGF 诱导OPCs 增殖[16] 。RSC96是永生化的大鼠来源的雪旺细胞株[17-18] ,用其制备的条件培养液(RSC96-CM)是否也像B104CM一样可促进OPCs 的增殖尚不清楚,如果可以,将找到除B104CM以外的另一个简单易制备的OPCs体外扩增剂,为OPCs体外生物学特性和临床细胞移植治疗神经退行性疾病等研究提供大量的细胞来源。因此,在本研究中,我们进一步观察RSC96的条件培养液(RSC96-CM)对OPCs增殖的影响,并探讨了其作用机制。
1 材料和方法 1.1 材料和试剂SD孕鼠(上海杰思捷实验动物有限公司,合格证编号为2010002603672)。主要试剂:DMEM/F12、bFGF、TRIzol 为美国Invitrogen 公司产品,PDGF-AA、AG1295、ELISA kits为美国R & D 公司产品,Hoechst 33342、PD173074、U0126、SP600125 为Sigma 公司产品,Anti-A2B5 抗体、Anti-NG2抗体、Anti-GFAP抗体均购自Chemincon公司,Anti-Rip抗体由美国印第安纳大学徐晓明教授惠赠,荧光素标记的二抗购自Jackson ImmunoResearch公司,引物合成来自日本TakaRa公司,其他试剂均为国产分析纯。
1.2 实验方法 1.2.1 OPCs的分离、培养和鉴定按照课题组分离OPCs的常规方法[10-12, 15-16] ,使用A2B5抗体从胚胎15 d的SD大鼠脊髓分离出OPCs,OPCs 种于多聚赖氨酸Poly-D-lysine(PDL)包被的10×12 cm 培养皿内,培养液包含DMEM/F12、1 ×N2、1 ×B27、PDGF-AA(10 ng/mL)和bFGF(20 ng/mL)。5~7 d 后细胞传代,所有实验均使用第2 次传代(P2)的OPCs。P2 代OPCs 种于PDL包被的盖玻片上,常规培养2~3 d 后鉴定OPCs的纯度;在无血清的DMEM/F12 中培养诱导OPCs 向OLs 分化,在含10%血清的DMEM/F12 中培养诱导OPCs向Ⅱ型星型胶质细胞分化,5 d 后鉴定OPCs的双向分化潜能。以PBS 洗涤细胞后用4%的多聚甲醛固定细胞,细胞加封闭液(含10% 正常驴血清,0.3%Trixton-X100 的PBS),室温封闭1 h 后再分别与一抗A2B5、NG2、Rip(均1∶100)和GFAP(1∶200)在4 ℃孵育过夜,第2 天再分别与罗丹明或异硫氰酸荧光素标记的二抗在37 ℃孵育1 h。洗涤后,用含有Hoechst33342 的封片液封片后在Olympus IX71 荧光显微镜下观察拍照。
1.2.2 RSC96 的条件培养液(RSC96-CM)的制备RSC96细胞系培养于含10%胎牛血清的(FCS)的DMEM中。为制备RSC96-CM,以Hank's 液洗涤培养的RSC96 细胞两次,并改为无血清的DMEM继续培养。3 d后,收集培养上清,过滤后储存于-20 ℃冰箱中备用。
1.2.3 BrdU掺入实验P2代OPCs种于PDL包被的盖玻片上,常规培养1~2 d后撤除bFGF和PDGFAA,继续培养24 h,然后分别加入不同浓度比例(0,10%,30%,50% 和100%)的RSC96-CM和BrdU(10 μmol/L),16小时后固定细胞。为鉴定各种可能参与的信号途径,细胞在加入RSC96-CM前分别与AG1295(PDGFR信号的特异性抑制剂,5 μmol/L)、PD173074(bFGFR信号的特异性抑制剂,2 μmol/L)、U0126(ERK信号的特异性抑制剂,20 μmol/L)、SP600125(JNK信号的特异性抑制剂,20 μmol/L)预孵45 min。细胞固定后,以1 NHCl 37 ℃ 处理40 min 使DNA变性,加入BrdU(1∶80)和A2B5抗体(1∶200)4 ℃孵育过夜,再加入荧光标记的二抗37 ℃孵育1 h,封片后在Olympus IX71 荧光显微镜下观察拍照。
1.2.4 逆转录PCR(RT-PCR)按照试剂盒操作说明,应用TRIzol 试剂抽提RSC96细胞总RNA,并RNA 逆转录为cDNA,进行RT-PCR分析,以β-actin 作为内参照。根据PDGF-AA、bFGF和β-actin 的基因全长序列分别设计引物(TakaRa, Japan)。PDGF-AA引物:上游引物5'-TGTGCCCATCCGCAGGAAGAG-3',下游引物5'-TTGGCCACCTTGACACTGCG-3',长度225 bp;bFGF 引物:上游引物5'-GGCTTCTTCCTGCGCATCCA-3',下游引物5'-GCTCTTAGCAGACATTGGAAGA-3',长度353 bp;β-actin 引物:上游引物5'-ATTGTAACCAACTGGGACG-3',下游引物5'-TTGCCGATAGTGATGACCT-3',长度533 bp。
1.2.5 RSC96-CM中PDGF-AA和bFGF蛋白浓度的检测按照试剂盒操作说明,应用ELISA 试剂盒检测RSC96-CM中PDGF-AA和bFGF 蛋白的浓度,每个样本检测3次,取平均值。
1.3 统计学处理采用SPSS 16.0统计软件进行统计学分析。结果以均数±标准差的形式表示。组间比较采用单因素方差分析(One-Way ANOVA),两组之间比较采用t检验,P<0.05为差异有统计学意义。
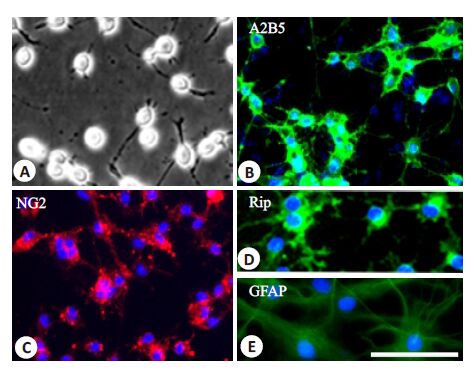
2 结果 2.1 OPCs培养和鉴定OPCs显示对称的双极或三极突起(图 1 A),表达特异性标志A2B5(图 1B)和NG2(图 1C)。在不含PDGF-AA和bFGF的无血清分化培养液中培养5d后,大多数细胞表达成熟少突胶质细胞的特异性标志Rip,并带有多极的细胞突起(图 1 D);但在含10%血清的分化培养液中培养5 d后,几乎所有的OPCs 分化为典型的纤维型星形胶质细胞形态,表达星形胶质细胞特异性标志GFAP(图 1E)。
|
图 1 OPCs的形态学和免疫细胞化学鉴定 Figure 1 Morphological and immunocytochemical characteristics of OPCs. A: Phase-contrast photomicrograph of OPCs cultured in growth medium; B: Cells that were immunopositive for A2B5 (green); C: Cells that were immunopositive for NG2 (Red); D: When cultured in FBS-free differentiation medium for 5 days, OPCs differentiated into Rip-positive oligodendrocytes (green); E: When cultured in differentiation medium containing 10% FBS for 5 days, OPCs differentiated into GFAP-positive type II astrocytes (green); Cells in B-E were counterstained with Hoechst 33342 (blue). Scale bar =25 μm. |
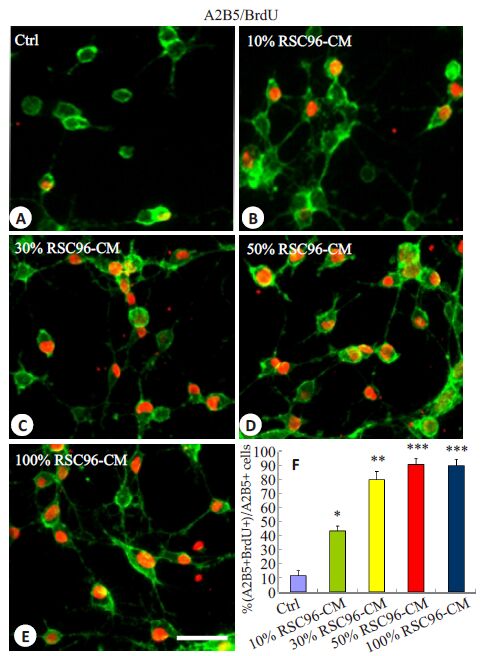
应用BrdU掺入实验检测OPCs的增殖,如图 2 所示,在含有10%、30%、50%和100% RSC96-CM的培养液中培养的OPCs,其BrdU + 细胞的比例分别为(43.49 ± 3.56)%,(80.18 ± 5.78)%,(91.02 ± 4.46)%和(90.20±4.29)%,与对照组相比(11.64±3.56)%均显著增加。其中,在含50% RSC96-CM 的培养液中培养的OPCs的BrdU+细胞的比例达高峰(图 2D,F)。
|
图 2 RSC96-CM诱导OPCs的增殖 Figure 2 RSC96-CM promotes the proliferation of cultured OPCs in vitro. A-E: Brdu-positive OPCs when the cells were cultured for 3 days in control (A), 10% (B), 30% (C), 50% (D) and 100% (E) RSC96-CM. F: Quantitative analysis of BrdU + cells in A2B5 + OPCs. Data presented are Mean ± SD (n=5). *P<0.05, **P<0.01, ***P<0.001 vs control. Scale bar =25 μm. |
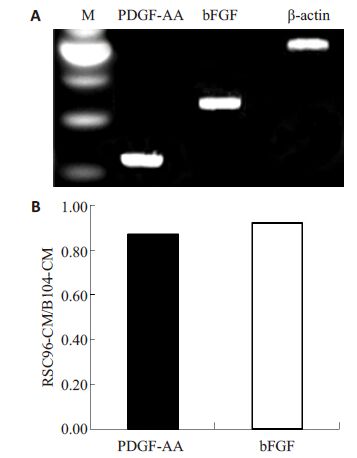
应用RT-PCR分析,我们证实RSC96细胞显著表达PDGF-AA和bFGF的mRNAs(图 3A)。我们进一步应用ELISA 检测了RSC96-CM中PDGF-AA和bFGF 的蛋白水平,发现PDGF-AA和bFGF的蛋白浓度分别达到20.42±3.88 ng/mL和11.94±2.85 ng/mL,分别是我们前期报道的B104CM中PDGF-AA和bFGF的蛋白水平的0.87倍和0.92倍(图 3B)。
|
图 3 PDGF-AA 和bFGF mRNA在RSC96细胞的表达及其蛋白在RSC96-CM中的水平 Figure 3 Expressions of PDGF-AA and bFGF mRNAs in RSC96 cells (A) and their protein concentrations in RSC96-CM determined by ELISA (B). |
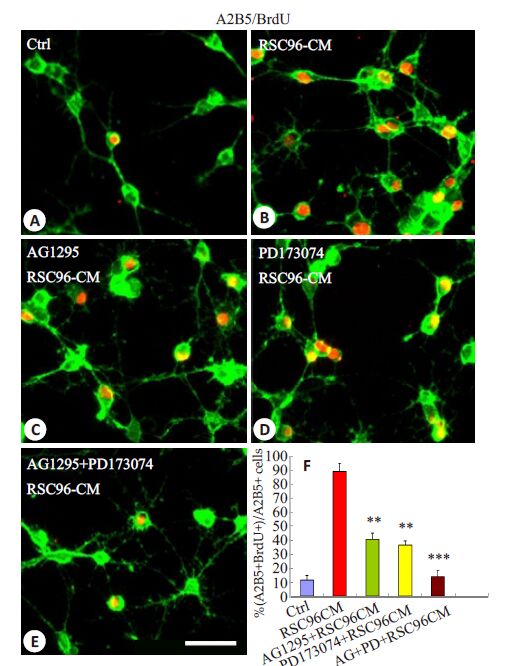
为鉴定是否PDGF-AA 和bFGF 是介导RSC96-CM诱导的OPCs增殖的关键因子,我们观察了PDGFR信号的特异性抑制剂AG1295 和bFGFR特异性抑制剂PD173074 能否阻断或降低RSC96-CM 诱导的OPCs增殖。结果表明,AG1295 和PD173074 均显著降低RSC96-CM诱导的OPCs增殖,BrdU+细胞的比例从89.05±5.76%(图 4B)分别减低到40.59±4.29%(图 4C,F;P<0.01)和36.28 ± 3.25%(图 4D,F;P<0.01)。此外,AG1295和PD173074 的联合应用几乎阻断了所有OPCs 的增殖(图 4E,F;P<0.001)。这些结果表明,PDGF-AA和bFGF是介导RSC96-CM诱导的OPCs增殖的关键因子。
|
图 4 PDGF-AA 和bFGF抑制剂对RSC96-CM诱导的OPCs增殖的影响 Figure 4 Effects of the inhibitors of PDGF-AA and bFGF on RSC96-CM-induced OPC proliferation. A-E: Effects of pre-incubationwith AG1295 (C), PD173074 (D) or AG1295 + PD173074 (E) onOPC proliferation induced by RSC96-CM analyzed using BrdUincorporation assay; F: Quantification of Brdu-positive OPCs (n=5). **P<0.01, ***P<0.001 vs control. Scale bar=25 μm. |
结果表明,Erk1/2 特异性抑制剂U0126 和JNK特异性抑制SP600125 的预孵OPCs后,RSC96-CM诱导的BrdU+ OPCs的比例从89.95%(图 5B,F;P<0.01)分别降低到30.32%(图 5C,F;P<0.01)和35.25%(图 5D,F;P<0.01)。此外,U0126和SP600125的联合应用几乎阻断了所有OPCs的增殖(图 5E,F;P<0.001)。这些结果表明,RSC96-CM通过激活Erk1/2 和JNK信号途径诱导OPCs增殖。
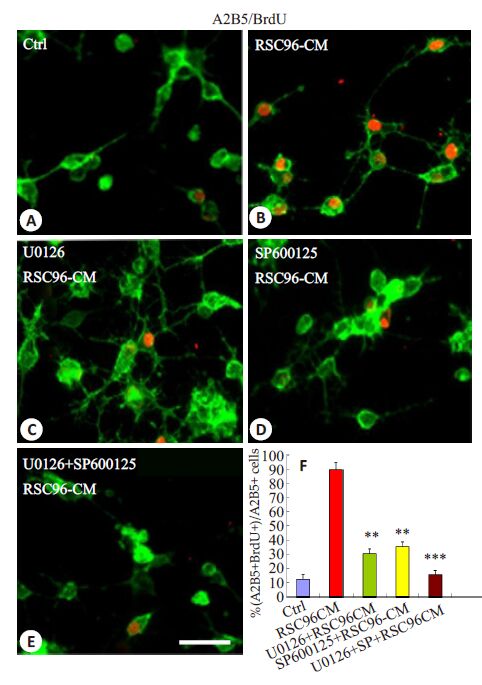
|
图 5 Erk1/2 和JNK 信号途径的抑制剂对RSC96-CM 诱导的OPCs增殖的影响 Figure 5 Inhibitors of Erk1/2 and JNK signaling blocked proliferation of OPCs induced by RSC96-CM. A-E: Effects of pre-incubation with U0126 (C), SP600125 (D) or U0126 + SP600125 (E) on OPC proliferation induced by RSC96-CM analyzed using BrdU incorporation assay; F: Quantification of Brdu-positive OPCs (n=5). **P<0.01, ***P<0.001 vs control. Scale bar =25 μm. |
我们的前期研究发现,大鼠SCs及其培养上清可在体内外诱导OPCs 增殖[15] ,其机制是通过分泌PDGF-AA和bFGF发挥作用[16] 。在本研究中,我们探讨了大鼠雪旺细胞株RSC96-CM对OPCs增殖的影响,以期找到另外一种类似B104CM的简便、易得、经济的OPCs体外培养扩增的刺激剂。
我们从胚胎15 d的大鼠脊髓分离培养了OPCs,证明几乎全部表达OPCs特异性表面标志A2B5和NG2,这些细胞在不同条件下可分化为成熟少突胶质细胞和纤维型星形胶质细胞,具有其特有的分化潜能,证明其有较高的纯度。并检测了RSC96-CM对OPCs增殖的影响,结果证实不同浓度的RSC96-CM均不同程度地明显促进OPCs的增殖,尤其当加入50%的RSC96-CM时增殖作用最为显著。这个结果表明,RSC96-CM与B104CM、SCM一样,同样具有促进OPCs增殖的作用。
PDGF-AA和bFGF是OPCs 增殖的重要丝裂原[4, 19] ,我们先前的报道证明B104 细胞和SCs 均通过分泌PDGF-AA和bFGF 诱导OPCs增殖[9] ,推测RSC96细胞也通过相同的机制来发挥作用。RSC96细胞同样显著表达PDGF-AA和bFGF的mRNA,其蛋白水平在RSC96-CM中浓度也较高,接近其在B104CM中的浓度。因为OPCs 同样表达PDGF-AA 和bFGF 的受体[9, 20-21] 。AG1295[17, 22-24] 和PD173074[25-28] 分别是PDGFR 和bFGFR 信号途径的特异性抑制剂,本研究中应用AG1295和PD173074分别阻断PDGFR和bFGFR信号途径,结果发现AG1295 和PD173074 均显著降低RSC96-CM诱导的OPCs增殖,尤其是两者联合使用,几乎阻断了所有的OPC增殖,这些结果表明,虽然与B104-CM相比,RSC96-CM中PDGF-AA和bFGF的蛋白水平稍低,但足以刺激OPCs的增殖,与B104细胞和SCs 一样,RSC96 细胞也是通过分泌PDGF-AA 和bFGF 诱导OPCs增殖。
Erk1/2、JNK等丝裂原激活的蛋白激酶(MAPKs)的活化是细胞生长、分化和针对各种外部刺激最迅速的反应事件,在细胞的生长、增殖等活动中起重要作用[29] 。我们先前的研究发现,B104CM诱导OPCs的增殖的过程要求激活ERK[10] 和JNK[11] 信号途径。本实验中,我们同样发现,Erk1/2和JNK信号途径被相应的抑制剂阻断后,RSC96-CM诱导的OPCs增殖明显减低。这些结果表明,RSC96-CM通过启动Erk1/2和JNK信号途径诱导OPCs增殖。
综上所述,我们的研究证实,RSC96-CM具有显著促进OPCs的增殖,其作用机制是通过RSC96分泌的PDGF-AA和bFGF激活Erk1/2和JNK信号途径。
由于RSC96 细胞系培养简单,RSC96-CM 像B104CM一样容易制备,RSC96-CM也可以作为OPCs常规培养的扩增剂,这为OPCs体外培养、扩增找到了另一种新的替代物,为OPCs体外生物学特性和临床细胞移植治疗神经退行性疾病等研究提供大量的细胞来源。
| [1] | Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system[J]. Physiol Rev, 2001, 81 (2): 871-927. |
| [2] | Iglesias-Rozas JR, Garrosa M. The discovery of oligodendroglia cells by Rio-Hortega:his original articles. 1921[J]. Clin Neuropathol, 2012, 31 (6): 437-9. |
| [3] | Eftekharpour E, Karimi-Abdolrezaee S, Fehlings MG. Current status of experimental cell replacement approaches to spinal cord injury[J]. Neurosurg Focus, 2008, 24 (3/4): E19. |
| [4] | Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium[J]. Nature, 1983, 303 (5916): 390-396. DOI: 10.1038/303390a0. |
| [5] | Qi Q, Zhang YX, Shen L, et al. Olig1 expression pattern in neural cells during rat spinal cord development[J]. Neuropsychiatr Dis Treat, 2016, 12 : 909-16. |
| [6] | Yang JL, Cheng XE, Shen JX, et al. A novel approach for amplification and purification of mouse oligodendrocyte progenitor cells[J]. Front Cell Neurosci, 2016, 10 : 203. |
| [7] | Louis JC, Magal E, Muir D, et al. CG-4, a new bipotential glial cell line from rat brain,is capable of differentiating in vitro into either mature oligodendrocytes or type-2 astrocytes[J]. J Neurosci Res, 1992, 31 (1): 193-204. DOI: 10.1002/(ISSN)1097-4547. |
| [8] | Fu SL, Hu JG, Li Y, et al. Induction of rat neural stem cells into oligodendrocyte precursor cells[J]. Sheng Li Xue Bao, 2005, 57 (2): 132-8. |
| [9] | Hu JG, Wu XJ, Feng YF, et al. PDGF-AA and bFGF mediate B104CM-induced proliferation of oligodendrocyte precursor cells[J]. Int J Mol Med, 2012, 30 (5): 1113-8. |
| [10] | Hu JG, Wu XJ, Feng YF, et al. The molecular events involved in oligodendrocyte precursor cell proliferation induced by the conditioned medium from B104 neuroblastoma cells[J]. Neurochem Res, 2013, 38 (3): 601-9. DOI: 10.1007/s11064-012-0957-0. |
| [11] | Hu JG, Wu XJ, Feng YF, et al. JNK is necessary for oligodendrocyte precursor cell proliferation induced by the conditioned medium from B104 neuroblastoma cells[J]. J Mol Neurosci, 2014, 52 (2): 269-76. DOI: 10.1007/s12031-013-0135-0. |
| [12] | Oudega M, Xu XM. Schwann cell transplantation for repair of the adult spinal cord[J]. J Neurotrauma, 2006, 23 (3/4): 453-67. |
| [13] | Kim S, Maynard JC, Sasaki Y, et al. Schwann cell O-GlcNAc glycosylation is required for myelin maintenance and Axon integrity[J]. J Neurosci, 2016, 36 (37): 9633-46. DOI: 10.1523/JNEUROSCI.1235-16.2016. |
| [14] | Jessen KR, Mirsky R. The repair Schwann cell and its function in regenerating nerves[J]. J Physiol, 2016, 594 (13): 3521-31. DOI: 10.1113/JP270874. |
| [15] | Hu JG, Wang XF, Deng LX, et al. Cotransplantation of glial restricted precursor cells and Schwann cells promotes functional recovery after spinal cord injury[J]. Cell Transplant, 2013, 22 (12): 2219-36. DOI: 10.3727/096368912X661373. |
| [16] | Chen YJ, Zhang JX, Shen L, et al. Schwann cells induce Proliferation and Migration of Oligodendrocyte Precursor Cells Through Secretion of PDGF-AA and FGF-2[J]. J Mol Neurosci, 2015, 56 (4): 999-1008. DOI: 10.1007/s12031-015-0570-1. |
| [17] | Badache A, De Vries GH. Neurofibrosarcoma-derived Schwann cells overexpress platelet-derived growth factor(PDGF)receptors and are induced to proliferate by PDGF BB[J]. J Cell Physiol, 1998, 177 (2): 334-342. DOI: 10.1002/(ISSN)1097-4652. |
| [18] | Lu MC, Lai TY, Hwang JM, et al. Proliferationand migrationenhancing effects of ginseng and ginsenoside Rg1 through IGF-Iand FGF-2-signaling pathways on RSC96 Schwann cells[J]. Cell Biochem Funct, 2009, 27 (4): 186-192. DOI: 10.1002/cbf.v27:4. |
| [19] | Asakura K, Hunter SF, Rodriguez M. Effects of transforming growth factor-beta and platelet-derived growth factor on oligodendrocyte precursors: Insights gained from a neuronal cell line[J]. J Neurochem, 1997, 68 (6): 2281-90. |
| [20] | Li N, Leung GK. Oligodendrocyte precursor cells in spinal cord injury: a review and update[J] . 2015: 235195. |
| [21] | Franco PG, Pasquini JM, Silvestroff L. Optimizing culture medium composition to improve oligodendrocyte progenitor cell yields in vitro from subventricular Zone-Derived neural progenitor cell neurospheres[J]. PLoS One, 2015, 10 (4): e0121774. DOI: 10.1371/journal.pone.0121774. |
| [22] | Jiang ZY, Zhong GQ, Wen LA, et al. The role of Platelet-Derived growth Factor-B/Platelet-Derived growth factor receptor-beta signaling in chronic atrial fibrillation[J]. Cardiology, 2016, 133 (4): 242-56. DOI: 10.1159/000442940. |
| [23] | Pascuali N, Scotti L, Abramovich D. Inhibition of platelet-derived growth factor(PDGF)receptor affects follicular development and ovarian proliferation, apoptosis and angiogenesis in prepubertal eCG-treated rats[J]. Mol Cell Endocrinol, 2015, 412 : 148-58. DOI: 10.1016/j.mce.2015.04.021. |
| [24] | Banai S, Wolf Y, Golomb G, et al. PDGF-receptor tyrosine kinase blocker AG1295 selectively attenuates smooth muscle cell growth in vitro and reduces neointimal formation after balloon angioplasty in swine[J]. Circulation, 1998, 97 (19): 1960-9. DOI: 10.1161/01.CIR.97.19.1960. |
| [25] | Pardo OE, Latigo J, Jeffery RE, et al. The fibroblast growth factor receptor inhibitor PD173074 blocks small cell lung cancer growth in vitro and in vivo[J]. Cancer Res, 2009, 69 (22): 8645-51. DOI: 10.1158/0008-5472.CAN-09-1576. |
| [26] | Kumar SB, Narasu L, Gundla R. Fibroblast growth factor receptor inhibitors[J]. Curr Pharm Des, 2013, 19 (4): 687-701. DOI: 10.2174/138161213804581963. |
| [27] | Mohanan V, Temburni MK, Kappes JC, et al. L1CAM stimulates glioma cell motility and proliferation through the fibroblast growth factor receptor[J]. Clin Exp Metastasis, 2013, 30 (4): 507-20. DOI: 10.1007/s10585-012-9555-4. |
| [28] | Yang Y, Adachi K, Sheridan MA, et al. Heightened potency of human pluripotent stem cell lines created by transient BMP4 exposure[J]. Proc Natl Acad Sci U S A, 2015, 112 (18): E2337-46. DOI: 10.1073/pnas.1504778112. |
| [29] | Peti W, Page R. Molecular basis of MAP kinase regulation[J]. Protein Sci, 2013, 22 (12): 1698-710. DOI: 10.1002/pro.v22.12. |
 2017, Vol. 37
2017, Vol. 37

