Quanshi, MD, PhD, professor. E-mail:wqslph@163.net
A solitary pulmonary nodule (SPN) is defined as a single round or oval opacity within 3 cm in diameter detected in the lungs by an imaging modality. SPNs, which are completely surrounded by pulmonary parenchyma and not associated with lymphadenopathy, atelectasis, or pneumonia [1, 2] , are noted on approximately 2% of chest radiographs [3] . With the advent of computed tomography (CT), more SPNs have been detected [1, 4-7] and their early detection and accurate discrimination can be of much clinical importance to reduce lung cancer-specific mortality [8, 9] .
Fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) is becoming a front-line modality for the evaluation of SPNs by measuring glucose consumption of the lesion. A SPN with an increased 18F-FDG uptake that exceeds the level in the mediastinal blood pool or with a maximum standardized uptake value (SUVmax) ≥2.5 is often considered to be lung cancer [10, 11] . However, there is still controversy over the value of using PET alone to characterize SPNs [12-15] .
Previous studies reported that the combination of PET and CT improved the differential diagnosis of SPNs [12] . This one-stop diagnostic scheme utilizes CT images for registration of the PET images, but the poor quality of these images, resulting from the use of a low current (~80-130 mA) and a thick section (~3-5 mm), hampers the diagnostic accuracy [12] . The sequential thin-section CT (with 1-mm-thick sections) is capable of covering the entire nodule using single-breath-hold methodology, and thus produces a better image quality and provides more diagnostic information than the routine thick-section CT [16-18] . We hypothesized that the functional information from 18F-FDG PET/CT combined with the excellent anatomical details from regional thin-section CT images helps to improve the diagnostic accuracy for SPNs. But so far, no studies have been reported to test this hypothesis. Therefore, we carried out this present study and performed additional regional thin-section CT scan for every SPN after a routine PET/ CT scan to investigate the performance of the two modalities combined in the characterization of SPNs.
PATIENTS AND METHODS PatientsThis study was carried out with approval from the Ethical Committee of the Institutional Review Board of Nanfang Hospital, Southern Medical University. Due to the retrospective nature of the study, the requirement of informed consent was waived.
The patients were identified upon a retrospective review of the PET center database at our hospital. Between January, 2007 and March, 2014, 267 patients (including 168 male and 99 female patients with an average age of 59 ± 11 years, range 32-87 years) were referred to 18F-FDG PET/CT for evaluation of SPNs detected by routine CT scans, which failed to determine the nature of the lesions. None of patients received anticancer treatment prior to the examinations. Twenty-six of the enrolled patients had diabetes mellitus, which was well controlled with diabetic therapy. 18F-FDG PET/CT was performed within one month since the lesions had been initially found on CT. All the malignant lesions were diagnosed by pathology. Benign lesions were confirmed by pathology following surgery or biopsy (63 cases) or in clinical follow-up (25 cases). The follow-up duration was>2 years (mean 1241±362 days).
PET/CT image acquisitionTwo-hundred and two patients received PET/CT examination using a Discovery LS PET/CT scanner (GE Healthcare, USA), and 65 patients were examined using a Biograph mCTx scanner (Siemens, Germany). The patients were instructed to fast for at least 6 h prior to the PET/CT scan; none of the patients had a blood glucose level >7 mmol/L before scanning.
Approximately 60 min after an intravenous injection of 5.55 MBq/kg of 18F-FDG, whole-body PET/ CT was performed according to previously reported guidelines for tumor imaging with 18F-FDG PET/CT and following the established protocols in our center [19-21] .
Thin-section CT image acquisitionThin-section CT of each nodule was performed immediately after the completion of PET/CT scan using a single-breath-hold technique on asyn-modality CT. The acquisition parameters were 140 kVp, 160 mAs, 0.875 pitch, and 0.625 mm collimation. No intravenous contrast was used. After scanning, the thin-section CT images were reconstructed into 1.0-mm-thick sections using high-frequency algorithms. The diameter of each lesion was measured on a transaxial lung window CT image.
PET image interpretationAll the PET/CT images were independently read by two nuclear medicine physicians with over 5 years of experience in nuclear medicine, both blinded to the thin-section CT findings. The location of each nodule was recorded on the CT images. A nodule with a 18F-FDG uptake that was higher than that in the mediastinal blood pool was considered suspicious of cancer by visual analysis. For semi-quantitative analysis, a region of interest (ROI) was drawn along the margin of each lesion to measure the SUVmax. In SPNs with negative PET findings, the ROIs were drawn on the CT images and copied to the corresponding regions on the PET images.
Thin-section CT image interpretationThe thin-section CT images were interpreted by two experienced radiologists who were blinded to the PET imaging findings. On the thin-section CT images, the nodules were classified as ground-glass nodules (GGNs), solid nodules or mixed nodules according to the established criteria [6, 20] . On the basis of thin-section CT features such as attenuation, size, calcification, fat, edge characteristics (irregularity, spiculation, lobulation, and smooth and well-defined margin), presence of air bronchogram, cavitation, feeding vessel signs, or pleural tails, the 5-point Likert scale was used to represent the likelihood of a lesion to be malignant. The SPNs were scored as follows: 1=not suspicious for malignancy (e.g., soft tissue, round, smooth and well-defined margins, and calcified); 2=low suspicion; 3=intermediate suspicion (e. g., mixed or soft-tissue attenuation, non-calcified, smooth margins, and >10 mm in diameter); 4= moderately high suspicion; and 5=high suspicion (e.g., mixed attenuation with a central zone of high attenuation, lobulation, spiculation, poorly defined, air bronchogram, feeding vessel sign, and >10 mm in diameter) [6, 11, 12, 16-18].
PET/CT image interpretationTwo criteria were used to diagnose lung cancer based on only 18F-FDG uptake in the PET findings (Criterion 1) or on both PET and thin-section CT findings (Criterion 2).
For Criterion 2, the consistency between thinsection CT and PET interpretations indicated that the PET/CT classification did not differ from that by thinsection CT or PET. For the nodules where inconsistency arose in PET and thin-section CT ratings, the lesion was further analyzed by a protocol similar to a previously established one [12] . A lesion was considered malignant or benign for a thin-section CT score of 5 (high suspicion) or 1 (not suspicious for malignancy), respectively, regardless of 18F-FDG uptake. For a lesion with a thin-section CT score of 2, 3, or 4, the diagnosis was further determined by PET (i.e., a lesion with positive PET results was considered malignant, and one with negative PET results was considered benign).
Statistical analysisSPSS 16.0 software (SPSS, Inc., Chicago, IL) was used for all analyses. The data of the continuous variables were expressed as Mean ±SD. Student's t-test was used to compare the parameters between the subgroups. To evaluate the statistically significant differences in the sensitivity, specificity, and accuracy of the diagnostic methods, 95% confidence intervals (95% CI) were calculated. Pearson's Chi-square test and Fisher's exact test were used to compare the categorical variables between groups. A P value less than 0.05 was considered to indicate a statistically significant difference.
RESULTS SPN pathology and clinical follow-upOf the SPNs in the 267 patients included, 179 were diagnosed as lung cancer as confirmed by pathological examination of the resected specimens. The lung cancer diagnoses included adenocarcinoma in 145 cases, squamous cell carcinoma in 22 cases, neuroendocrine tumor in 4 cases, carcinosarcoma in 2 cases, mucous epidermoid carcinoma in 2 cases, lymphatic carcinoma in 3 cases, and clear-cell carcinoma in 1 cases.
Eighty-eight SPNs were diagnosed as benign lesions, including tuberculosis in 36 cases, inflammatory pseudotumor in 8 cases, pulmonary hamartoma in 10 cases, sclerosing hemangioma in 6 cases, cryptogenic organizing pneumonia in 5 cases, fungal infection in 6 cases, nonspecific bacterial infection in 8 cases, and unknown origin in 9 cases. The nine lesions of unknown origin were confirmed as benign on follow-up plain radiograph or CT images without pathological examination, and the lesions showed no detectable growth.
Comparison of the two criteria for evaluation of solid and non-solid SPNsFor lung cancer diagnosis for all the 267 SPNs, Criterion 1 yielded a diagnostic sensitivity, specificity, and accuracy of 80.4% (144/179), 68.1% (60/88), and 76.4% (204 /267), respectively (Tab. 1). Thirty-five SPNs had false-negative PET results, including 34 (97.1% ) well-differentiated adenocarcinomas and one (2.90% ) moderately differentiated adenocarcinoma. For lesions with diameters ≤1.0 cm, ≤2 cm and ≤3 cm, PET yielded false-negative results in 33.3% (3/9), 25% (20/ 81), and 13.3% (12/89) of the lesions, respectively, showing no significant differences in the false-negative rates among differently sized SPNs (χ2=4.809, P=0.090). Of the 35 lesions with false-negative PET results, 11 (31.4% ) were solid nodules and 24 (68.6% ) were non-solid nodules. PET yielded false-positive results for 28 lesions caused by active tuberculosis in 10 cases, nonspecific bacterial infection in 7 cases, inflammatory pseudotumor in 5 cases, sclerosing hemangioma in 4 cases, and fungal infection in 2 cases.
| Table 1 Diagnostic performance of the two criteria for solid SPNs, non-solid SPNs and total SPNs |
By comparison, thin-section CT provided greater diagnostic information for the SPNs in 225 of the patients (84.2% ), and displayed more clearly such imaging features as calcification, cavitation, fat, lobulation, air bronchogram, and feeding vessels. Eighteen cancerous nodules were accurately identified as mixed nodules by thin-section CT, which were previously considered solid nodules on thick-section CT. Criterion 2 was more sensitive than Criterion 1 in diagnosing lung cancer (91.0% vs 80.4%; χ2=8.254, P= 0.004) with also a greater accuracy (87.2% vs 76.4%; χ2= 5.885, P=0.017). However, no significant difference in specificity was observed between Criterion 2 and Criterion 1 (79.5% vs 68.1% ; χ2=2.943, P=0.086) (Tab. 1). Criterion 2 improved the diagnostic accuracy in 29 SPNs (19 lung cancers and 10 benign lesions) (Fig. 1-3).
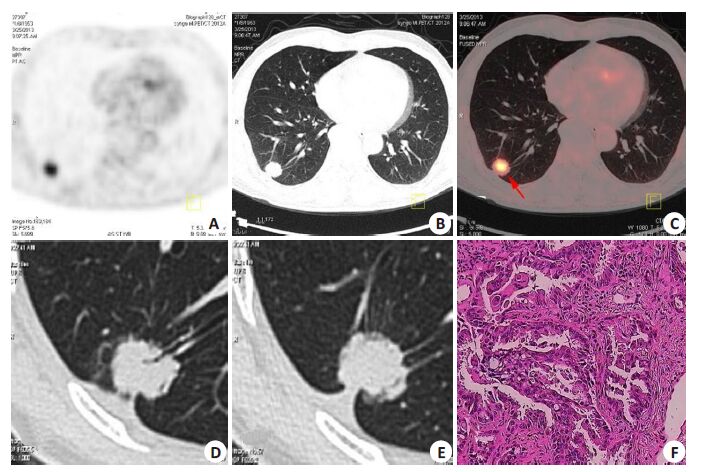
|
Figure 1 A 61-year-old man was found to have a SPN in the lower right lung 5 days prior to admission. 18F-FDG PET/CT was performed for further evaluation. 18F-FDG PET/CT revealed a SPN (size, 2.1 cm×1.8 cm) in the lower right lung (A). 18F-FDG uptake within the lesion was intense (higher than that in the mediastinal blood pool ), with an SUVmax of 5.1(B, C), which indicated that the lesion was lung cancer according to Criterion 1. An additional thin-section CT (D, E) showed that the lesion was a solid SPN with signs of lobulation, air bronchogram, feeding vessel, and pleural tail, which were also indicative of lung cancer. The diagnoses were consistent between the two criteria. The patient received a pulmonary lobectomy, and a diagnosis of adenocarcinoma was established by pathology (F, HE staining, original magnification: ×200). |
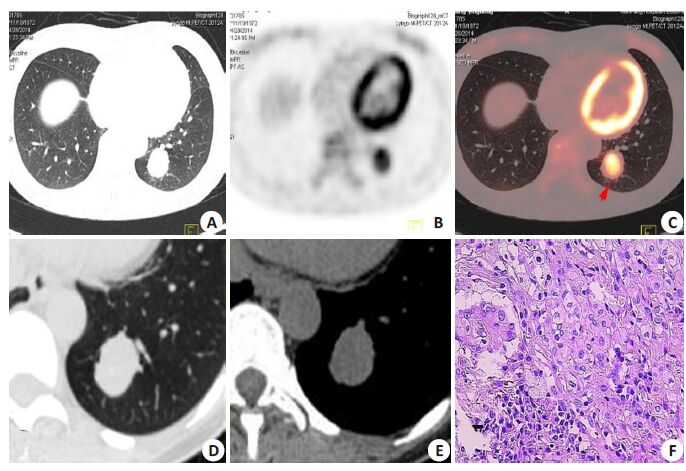
|
Figure 2 A 41-year-old woman with a SPN detected by CT 15 days prior to admission. 18F-FDG PET/ CT revealed a solid SPN (2.5 cm × 2.8 cm) in the lower left lung (A). The lesion had increased 18F-FDG uptake with a SUVmax of 4.8 (B, C), for which a diagnosis of lung cancer was made according to Criterion 1. However, thin-section CT (D, E) showed smooth and well defined margin of the lesion without malignant signs of lobulation, spiculation, poorly defined margin, air bronchogram, or feeding vessels. The nodule was diagnosed as a benign lesion according to Criterion 2, and was confirmed as a sclerosing hemangioma at subsequent lobectomy (F, HE staining, ×400). |
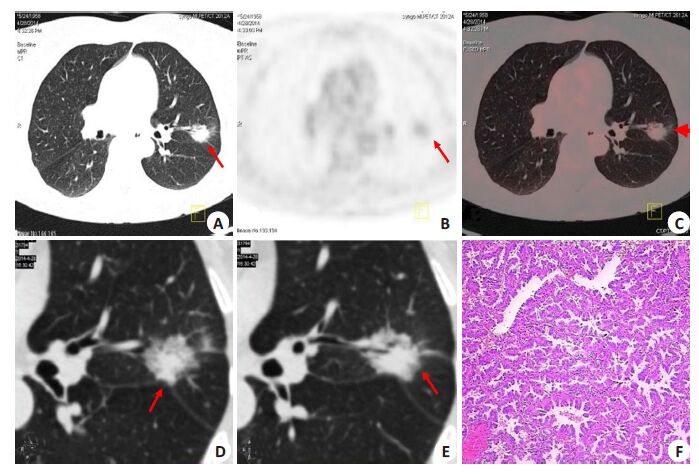
|
Figure 3 A representative case in a 55-year-old man complaining of cough and expectoration for 10 days. Chest CT showed a solid SPN in the upper left lung, which did not respond to treatment with antibiotics for two weeks. 18F-FDG PET/CT revealed a SPN of 2.0 cm × 1.8 cm (A) in the upper left lung with low 18F-FDG uptake (SUVmax=1.8; B, C, arrows). The lesion was considered benign according to Criterion 1. Further thin-section CT examination showed that the lesion was a mixed nodule with such malignant CT features as mixed attenuation with a central zone of high attenuation, lobulation, spiculation, poor definition, and air bronchogram (D, E), and the diagnosis of lung cancer was made according to Criterion 2. Lobectomy was performed, and the lesion was confirmed as a well-differentiated adenocarcinoma (F, HE staining, ×200). |
Of the 267 SPNs analyzed, 215 presented as solid nodules, including 139 (64.7%) lung cancer cases and 76 (35.3% ) benign cases. Lung cancer lesions had a higher average 18 F-FDG uptake than benign lesions with SUVmax of 7.36±4.31 vs 2.28±1.97, respectively (t= 9.710, P=0.000). The average size of malignant SPNs was also significantly larger than that of benign lesions (2.15±0.64 vs 1.84±0.70; t=3.298, P=0.001).
For evaluating solid SPNs, Criterion 1 and Criterion 2 showed similar diagnostic sensitivity (92.0% vs 91.3%, χ2=0.047, P=0.828) and accuracy (84.1% vs 87.4% ; χ2=0.936, P=0.333). Although 8 SPNs with increased uptake of 18F-FDG were diagnosed correctly as benign lesions by Criterion 2 (Fig. 2) and the specificity increased from 69.7% by Criterion 1 to 80.2% by Criterion 2, the two criteria showed no significant difference in the diagnostic specificity (χ2=2.246, P=0.134).
Comparison of the two criteria in evaluating non-solid SPNsOf the 267 SPNs, 52 were non-solid nodules, including 40 (76.9%) lung cancer lesions and 12 (23.1%) benign lesions. The cancerous lesions contained 8 GGNs and 32 mixed nodules. The average size of the lung cancer lesions was significantly larger than that of the benign lesions (2.05±0.54 vs 1.33±0.47 cm; t=4.142, P=0.000), but the malignant and benign lesions showed no significant difference in 18F-FDG uptake with SUVmax of 2.46±1.81 and 2.48±1.74, respectively (t=0.034, P= 0.973).
The non-solid SPNs presented with some features on thin-section CT images to distinguish benign from malignant lesions (Tab. 2). For instance, only 25% of the benign lesions had a diameter >1.5 cm, as compared to the rate of 82.5% in lung cancer lesions (P=0.000). The CT features of lobulation, air bronchogram, and feeding vessel were more commonly observed in malignant nodules (Tab. 2). The frequencies of spiculation and a pleural tail, however, showed no significant differences between malignant and benign lesions (P>0.05; Tab. 2).
| Table 2 CT features of lung cancer lesions (n=40) and benign lesions (n=12) in non-solid nodules |
For the non-solid lesions, the sensitivity and accuracy of Criterion 2 for lung cancer diagnosis were significantly higher than those of Criterion 1 (sensitivity: 90.0% vs 40.0%, P=0.000; accuracy: 86.5% vs 44.2%, χ2=22.562, P=0.000). However, no significant difference in the diagnostic specificity between the two criteria (75.2% vs 58.3%, P=0.667).
The use of thin-section CT rectified the diagnoses of 20 malignant lesions, which were originally diagnosed as benign lesions for a 18F-FDG uptake lower than that in the mediastinal blood pool (Fig. 3). Two benign lesions with increased 18F-FDG uptake were also correctly diagnosed as benign by thin-section CT.
DISCUSSIONPrevious studies have demonstrated the value of 18F-FDG PET/CT in characterization of SPNs [10, 11, 22] , and in the study by Fletcher et al, the diagnostic sensitivity and specificity of 18F-FDG PET for lung cancer could reach 91.7% and 82.3% in a cohort of 344 patients, respectively [11] . However, some studies suggested that the value of 18F-FDG PET/CT in evaluating SPNs had been overestimated [12-15, 23] , and its diagnostic sensitivity did not exceed 70% in characterizing SPNs [12, 24] .
In this study we confirmed the limitations of PET alone in evaluating SPNs, whose diagnostic specificity was only moderate (69.7%) for solid SPNs in spite of its high sensitivity (92.0% ) and accuracy (84.1% ). For characterizing non-solid nodules, the performance of PET alone deteriorated drastically with a rather low sensitivity (40.0% ), specificity (58.3% ), and accuracy (44.2% ). Non-solid cancerous nodules, which exhibit typically the slow-growth characteristics [25-28] , are liable to be mistakenly diagnosed as benign lesions by 18F-FDG PET [25-30] . With the widespread use of low-dose CT for lung scanning, an increasing number of non-solid nodules are detected, which poses a great challenge for PET/CT [3] .
CT is the traditional standard imaging modality for the detection and evaluation of SPNs [5-7] . CT images can be obtained during PET/CT scans, and the combination of PET and CT was reported to create a synergistic effect in the differential diagnosis of SPNs [12, 31] . However, the CT module in PET/CT, when compared to a conventional CT scan, acquires images often with a low current (~80-130 mAs); the CT images acquired from a PET/CT system are prone to breathing and motion artifacts, and its greater section thickness often fail to fully display the details of the lesion [12, 16-18] .
In contrast, thin-section CT scanning can enhance the image quality and provide more and clearer imaging details of the SPNs [16-18] . In our cases, we obtained more diagnostic information of the SPNs using thin-section CT in 225 of the patients (84.2% ), and 18 cancerous nodules that had been previously diagnosed as solid nodules were accurately affirmed as mixed nodules. In cases of non-solid SPNs, we found that lesion size and the thin-section CT findings of lobulation, air bronchogram, and feeding vessel all helped in the differential diagnoses, as was consistent with previous studies [16-18] . Thin-section CT rectified the diagnoses of 50% (20/40) of the cancerous lesions, which were originally diagnosed as benign lesions by PET. The Criterion 2, which combined 18F-FDG PET/CT and thin-section CT, showed significantly improved performance for lung cancer diagnosis of the non-solid lesions as compared with the Criterion 1 (PET/CT alone) with a sensitivity and accuracy reaching 90.0% and 86.5% , respectively. Nevertheless, the performance of this combined modality was not as good for solid nodules, and the increase in the diagnostic specificity (from 69.7% to 80.2%) for nodule characterization was not statistically significant.
This study has some limitations due to its retrospective nature and the small sample size of the patients with non-solid nodules, especially those with benign non-solid nodules (only 12 patients). Further studies are warranted to further confirm our results.
CONCLUSIONWe confirmed the limitations of PET alone in evaluating malignant non-solid nodules in terms of its potential to yield false-negative results. The combination of thinsection CT and PET can provide additional diagnostic information for evaluating SPNs, especially for non-solid ones. We recommend that regional thin-section CT scanning should be performed after routine PET/CT, especially when the patient has not undergone either of the examinations.
| [1] | Jeong YJ, Lee KS, Kwon OJ. Diagnosis and management of solitary pulmonary nodules[J]. Expert Rev Respir Med,2008, 2 (6) : 767-77. DOI: 10.1586/17476348.2.6.767. |
| [2] | Truong MT, Ko JP, Rossi SE, et al. Update in the evaluation of the solitary pulmonary nodule[J]. Radio Graphics,2014, 34 (6) : 1658-79. |
| [3] | Gómez-Sáez N1, González-álvarez I, Vilar J, et al. Prevalence and variables associated with solitary pulmonary nodules in a routine clinic-based population: a cross-sectional study[J]. Eur Radiol,2014, 24 (9) : 2174-82. DOI: 10.1007/s00330-014-3249-z. |
| [4] | Tsutani Y, Miyata Y, Nakayama H, et al. Appropriate sublobar resection choice for ground glass opacity-dominant clinical stage IA lung adenocarcinoma: wedge resection or segmentectomy[J]. Chest,2014, 145 (1) : 66-71. DOI: 10.1378/chest.13-1094. |
| [5] | Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines[J]. Chest,2013, 143 (5 Suppl) : e93-e120S. |
| [6] | Winer-Muram HT. The solitary pulmonary nodule[J]. Radiology,2006, 239 : 34-49. DOI: 10.1148/radiol.2391050343. |
| [7] | Truong MT, Sabloff BS, Ko JP. Multidetector CT of solitary pulmonary nodules[J]. Thorac Surg Clin,2010, 20 (1) : 9-23. DOI: 10.1016/j.thorsurg.2009.12.002. |
| [8] | Guerrera F, Errico L, Evangelista A, et al. Exploring stage I nonsmall- cell lung cancer: development of a prognostic model predicting 5-year survival after surgical resection[J]. Eur J Cardio Surg,2015, 47 (6) : 1037-43. DOI: 10.1093/ejcts/ezu410. |
| [9] | Kaiser F, Engelhardt M, Rawluk J, et al. Current treatment concepts of lung cancer[J]. Dtsch Med Wochenschr,2011, 136 (38) : 1901-6. DOI: 10.1055/s-0031-1286361. |
| [10] | Bar-Shalom R, Kagna O, Israel O, et al. Noninvasive diagnosis of solitary pulmonary lesions in cancer patients based on 2-fluoro-2- deoxy-D-glucose avidity on positron emission tomography/computed tomography[J]. Cancer,2008, 113 (11) : 3213-21. DOI: 10.1002/cncr.v113:11. |
| [11] | Fletcher JW, Kymes SM, Gould M, et al. A comparison of the diagnostic accuracy of 18F-FDG PET and CT in the characterization of solitary pulmonary nodules[J]. J Nucl Med,2008, 49 (2) : 179-85. DOI: 10.2967/jnumed.107.044990. |
| [12] | Kim SK, Allen-Auerbach M, Goldin J, et al. Accuracy of PET/CT in characterization of solitary pulmonary lesions[J]. J Nucl Med,2007, 48 (2) : 214-20. |
| [13] | Hashimoto Y, Tsujikawa T, Kondo C, et al. Accuracy of PET for diagnosis of solid pulmonary lesions with 18F-FDG uptake below the standardized uptake value of 2.5[J]. J Nucl Med,2006, 47 (3) : 426-31. |
| [14] | Alkhawaldeh K, Bural G, Kumar R, et al. Impact of dual-time-point (18)F-FDG PET imaging and partial volume correction in the assessment of solitary pulmonary nodules[J]. Eur J Nucl Med Mol Imaging,2008, 35 (2) : 246-52. DOI: 10.1007/s00259-007-0584-1. |
| [15] | Li S, Zhao B, Wang X, et al. Overestimated value of (18)F-FDG PET/ CT to diagnose pulmonary nodules: analysis of 298 patients[J]. Clin Radiol,2014, 69 (8) : e352-7. DOI: 10.1016/j.crad.2014.04.007. |
| [16] | Jiang B, Takashima S, Miyake C, et al. Thin-section CT findings in peripheral lung cancer of 3 cm or smaller: are there any characteristic features for predicting tumor histology or do they depend only on tumor size[J]. Acta Radiol,2014, 55 (3) : 302-8. DOI: 10.1177/0284185113495834. |
| [17] | Gaeta M, Barone M, Russi EG, et al. Carcinomatous solitary pulmonary nodules: evaluation of the tumor-bronchi relationship with thin-section CT[J]. Radiology,1993, 187 (2) : 535-9. DOI: 10.1148/radiology.187.2.8475303. |
| [18] | Lee HY, Goo JM, Lee HJ, et al. Usefulness of concurrent reading using thin-section and thick-section CT images in subcentimetre solitary pulmonary nodules[J]. Clin Radiol,2009, 64 (2) : 127-32. DOI: 10.1016/j.crad.2008.09.003. |
| [19] | Delbeke D, Coleman RE, Guiberteau MJ, et al. Procedure guideline for tumor imaging with 18F-FDG PET/CT 1.0[J]. J Nucl Med,2006, 47 (5) : 885-95. |
| [20] | Kim TJ, Park CM, Goo JM, et al. Is there a role for FDG PET in the management of lung cancer manifesting predominantly as ground-glassopacity[J]. AJR Am J Roentgenol,2012, 198 (1) : 83-8. DOI: 10.2214/AJR.11.6862. |
| [21] | Zhou WL, Wu HB, Wang LJ, et al. Usefulness and pitfalls of F- 18-FDG PET/CT for diagnosing extramedullary acute leukemia[J]. Eur J Radiol,2016, 85 (1) : 205-10. DOI: 10.1016/j.ejrad.2015.11.019. |
| [22] | Gould MK, Maclean CC, Kuschner WG, et al. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis[J]. JAMA,2001, 285 (7) : 914-24. DOI: 10.1001/jama.285.7.914. |
| [23] | Kagna O, Solomonov A, Keidar Z, et al. The value of FDG-PET/CT in assessing single pulmonary nodules in patients at high risk of lung cancer[J]. Eur J Nucl Med Mol Imaging,2009, 36 (6) : 997-1004. DOI: 10.1007/s00259-009-1061-9. |
| [24] | van Gómez López O, García Vicente AM, Honguero Martínez AF, et al. (18) F-FDG-PET/CT in the assessment of pulmonary solitary nodules: comparison of different analysis methods and risk variables in the prediction of malignancy[J]. Transl Lung Cancer Res,2015, 4 (3) : 228-35. |
| [25] | Lee HY, Lee KS. Ground-glass opacity nodules: histopathology, imaging evaluation, and clinical implications[J]. J Thorac Imaging,2011, 26 (2) : 106-18. DOI: 10.1097/RTI.0b013e3181fbaa64. |
| [26] | Travis WD, Garg K, Franklin WA, et al. Evolving concepts in the pathology and computed tomography imaging of lung adenocarcinoma and bronchioloalveolar carcinoma[J]. J Clin Oncol,2005, 23 (14) : 3279-87. DOI: 10.1200/JCO.2005.15.776. |
| [27] | Raad RA, Suh J, Harari S, et al. Nodule characterization: subsolid nodules[J]. Radiol Clin North Am,2014, 52 (1) : 47-67. DOI: 10.1016/j.rcl.2013.08.011. |
| [28] | Seidelman JL, Myers JL, Quint LE. Incidental, subsolid pulmonary nodules at CT: etiology and management[J]. Cancer Imaging,2013, 13 (3) : 365-73. DOI: 10.1102/1470-7330.2013.9025. |
| [29] | Lee HY, Choi YL, Lee KS, et al. Pure ground-glass opacity neoplastic lung nodules: histopathology, imaging, and management[J]. AJR Am J Roentgenol,2014, 202 (3) : W224-33. DOI: 10.2214/AJR.13.11819. |
| [30] | Ambrosini V, Nicolini S, Caroli P, et al. PET/CT imaging in different types of lung cancer: an overview[J]. Eur J Radiol,2012, 81 (5) : 988-1001. DOI: 10.1016/j.ejrad.2011.03.020. |
| [31] | Kagna O, Solomonov A, Keidar Z, et al. The value of FDG-PET/CT in assessing single pulmonary nodules in patients at high risk of lung cancer[J]. Eur J Nucl Med Mol Imaging,2009, 36 (6) : 997-1004. DOI: 10.1007/s00259-009-1061-9. |
 2017, Vol. 37
2017, Vol. 37
