2. 雅安市妇幼保健院妇产科,四川 雅安 625000
2. Department of Gynecology and Obstetrics, Ya'an Maternal and Children's Healthcare Hospital, Ya'an 625000, China
脐带是获取临床治疗用间充质干细胞(MSCs)的重要途径,脐带间充质干细胞(UCMSCs)与骨髓、脂肪来源的MSCs一样,在免疫调节、损伤修复和退行性疾病等领域有广阔的应用潜力[1-3]。近年来多项研究证实脐带来源的MSCs能分泌多种生物活性物质,包括促生长因子、细胞因子、趋化因子等[4-5],可在体内外促进细胞增殖和血管形成[6-7]、支持神经再生与保护[7-8]、支持造血[1, 9]、调控免疫[1, 10]等。
视黄醇(维生素A)是一种常见的营养因子,在体内可转化为视黄醛和视黄酸,进而在动物胚胎发生和生长发育过程中发挥抗氧化、生殖机能形成、视力维持以及促进细胞分化等调控作用[11]。近年来研究表明视黄醇有不同于视黄醛和视黄酸的促细胞分化作用,其主要机制是通过上调Nanog、Oct4和Sox2的表达来增强胚胎干细胞(ESCs)[12-14]、雄性生殖干细胞(MGSCs)[15]等的自我更新能力并维持其未分化状态。对于UCMSCs,视黄醇(1 μmol/L)可增强其体外培养增殖活力,上调Nanog、PCNA和C-myc等基因表达[16]。有研究证实人UCMSCs转录表皮生长因子(EGF)、白血病抑制因子(LIF)、干细胞因子(SCF)和集落刺激因子1(CSF1)[9, 17],这些因子与造血干细胞和雄性生殖细胞等的自我更新与分化以及神经再生等密切相关[7-9, 18-19],但视黄醇能否影响UCMSCs转录相关因子,目前还未见报道。本实验分别在胎牛血清、血清替代品(KSR)的培养基中添加视黄醇(1 μmol/L),从基因和蛋白水平评价视黄醇对人UCMSCs 表达LIF、EGF、CSF1、SCF 的影响,为UCMSCs分离培养及其产物应用提供理论依据。
1 材料和方法 1.1 材料脐带样品由四川雅安某妇产医院提供,在产妇及家属知情同意后,无菌获取健康足月剖宫产新生儿脐带。视黄醇、胰蛋白酶、Ⅳ型胶原酶、DNA酶、DMEM/F12 购自Sigma,胎牛血清(FBS)、KSR购自Gibco;总RNA 快速提取试剂盒购自北京百泰克公司,PrimeScriptTM RT reagent Kit、RNA PCR Kit(AMV)Ver.3.0 等购自TaKaRa;CD29、CD44、CD105、CD31、CD34 一抗购自Ycbio,Oct4、Nanog、Sox2 一抗购自CST,SP免疫组化染色试剂盒购自北京中杉金桥公司;人EGF、人SCF、人白血病抑制因子LIF及人CSF1的酶联免疫吸附试验(ELISA)试剂盒购自Ycbio;RT-PCR扩增引物以GenBank 收录GAPDH、EGF、LIF、SCF 和CSF1 的cDNA序列为模板,用Prime 5.0 软件设计,由北京华大生物工程有限公司合成,引物序列和目的片段长度见表 1。
| 表 1 目的基因的引物信息 Table 1 Primers for amplification of the targeted genes |
无菌取足月剖宫产胎儿脐带3~5 cm,置于无菌PBS中带回,PBS充分漂洗以去除血污,剔除羊膜和脐血管,将组织剪成大小约1 mm×1 mm×1 mm的组织块,3~5倍体积Ⅳ型胶原酶(1 mg/L)溶液4 ℃孵育过夜,后用3~5 倍量0.25%胰蛋白酶-EDTA(0.2 mg/L)-DNA酶(1 mg/L)37 ℃孵育30 min以终止消化,离心收集细胞,用12% FBS DMEM/F12培养液悬浮制成单细胞悬液(2×105/mL)接种于细胞培养板,37 ℃饱和湿度培养,细胞增殖至85%汇合度时0.25%胰蛋白酶-EDTA(0.2 mg/L)消化传代,细胞传至3代后用于后续试验。
细胞传至3代,消化后以1×105/孔细胞接种于24孔培养板,分别用添加12% FBS(F)、12% FBS+1 μmol/L视黄醇(F+R)、15% KSR(K)和15% KSR+1 μmol/L视黄醇(K+R)的DMEM/F12培养液培养,每组设置3个复孔,培养3 d,收集细胞和培养上清用RT-PCR和ELISA分析细胞因子EGF、LIF、SCF和CSF1的表达。
1.2.2 人UCMSCs 免疫组化鉴定以CD29、CD44、CD105、CD31、CD34、Oct4、Sox2和Nanog作为一抗对分离细胞进行免疫组化分析,实验依SP免疫组化染色试剂盒说明书进行,即用4%多聚甲醛固定,3% H2O2孵育,山羊血清37 ℃封闭10 min,滴加一抗CD29(1∶100)、CD44(1∶50)、CD105(1∶200)、CD31(1∶200)、CD34(1∶200)4 ℃孵育过夜,生物素标记二抗孵育20 min,辣根酶标记链霉卵白素孵育15 min,DAB显色5~10 min,倒置显微镜下观察(阳性细胞着色呈蓝色,阴性细胞不着色),拍照记录结果。
1.2.3 RT-PCR分析EGF、LIF、SCF和CSF1基因的转录表达以上述培养3 d的细胞作为样品,利用RT-PCR分析EGF、LIF、SCF、CSF1以及内参GAPDH的mRNA转录水平,所有分析样品经血球计数板计数以保证用于后续处理的细胞数量一致。细胞总RNA提取、反转录和PCR反应操作按RNA快速提取试剂盒、PrimeScriptTMRT reagent Kit和RNA PCR Kit(AMV)Ver.3.0的试剂盒说明书进行;PCR反应条件:95 ℃预变性3 min,94 ℃变性30 s,退火30 s,72 ℃延伸1 min,30 个循环,最后72 ℃延伸10 min(温度见表 1);反应完毕后取扩增产物于10 g/L 琼脂糖凝胶电泳(90 mV,30 min),凝胶成像仪(GelDoc2000,美国BIO-RAD)观察并记录电泳图像。利用Quantity One软件将电泳图像中各目的条带灰度值换算为具体数值,与内参基因GAPDH电泳条带灰度值相比的比值为目的基因的mRNA相对表达量,计算公式为:目的基因mRNA相对含量=目的基因条带灰度值/GAPDH基因条带灰度值×100%。
1.2.4 ELISA检测EGF、LIF、SCF和CSF1 的蛋白表达以上述培养3 d的细胞和培养液作为样品,利用ELISA分析EGF、LIF、SCF、CSF1在细胞和培养上清液中的含量。细胞蛋白抽提物反复冻融,经离心取上清液为分析样品;细胞抽提物和培养上清液中细胞因子含量测定按各试剂盒说明书进行,利用酶标仪PR-521读取各样品光密度值(A),根据标准品浓度及其对应光密度值建立标准曲线,在标准曲线范围内依照各样品光密度值分别确定EGF、LIF、SCF、CSF1的含量。ELISA试剂盒检测范围分别为EGF 0.2~4 μg/L,CSF1 25~500 ng/L,LIF70~2000 ng/L,SCF 20~600 ng/L,低于试剂盒检测下限值不列出。
1.3 统计学分析各实验数据以均数±标准差表示,采用SPSS 16.0统计软件进行单因素方差分析,P<0.05为差异有统计学意义。
2 结果 2.1 人UCMSCs的分离培养与鉴定如图 1 所示人UCMSCs呈多角形或长梭形,生长致密时呈成纤维细胞样,平行或漩涡样排列;免疫组化染色结果显示MSC标志抗原CD29、CD44、CD105,以及ESCs标志抗原Oct4、Nanog、Sox2均染色阳性,而内皮细胞标记抗原CD31和造血干细胞标记抗原CD34染色阴性(图 1)。
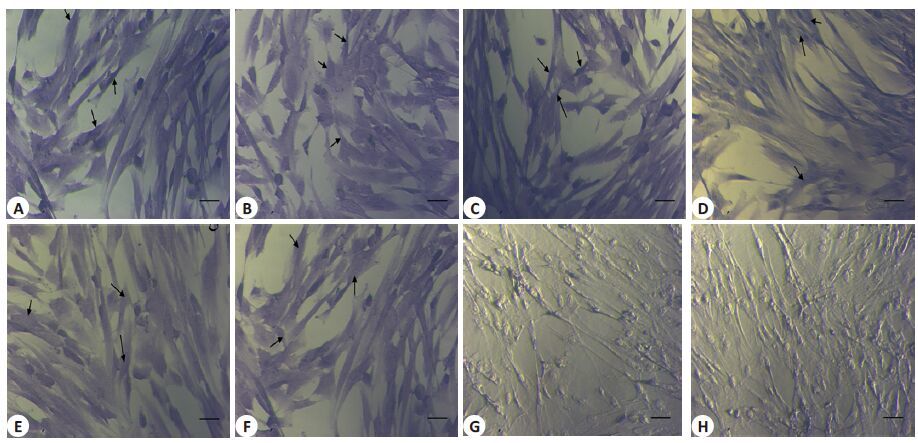
|
图 1 人UCMSCs免疫组化染色 Figure 1 Immunohematochemical staining of human UCMSCs (Scale bar: 50 μm). A-F: Positive staining results of CD29,CD44,CD105,Oct4,Nanog,and Sox2,respectively; G-H: Negative staining results of CD31 and CD34,respectively |
RT-PCR结果显示,所有培养条件下人UCMSCs均转录表达基因SCF、LIF、CSF1和EGF的mRNA(图 2);添加视黄醇(1 μmol/L),基因LIF、EGF的mRNA表达差异不显著(P>0.05),但显著提高CSF1 和SCF 的mRNA表达量(P<0.05,表 2)。
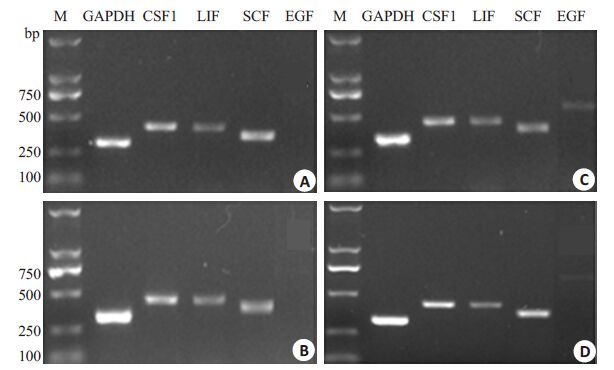
|
图 2 RT-PCR分析EGF、LIF、SCF和CSF1在人UCMSCs的表达 Figure 2 RT-PCR analysis of EGF, LIF, SCF and CSF1 expression in human UCMSCs.A-D: RT-PCR results for human UCMSCs cultured with 12% FBS, 12% FBS +retinol, 15% KSR, and 15% KSR+ retinol, respectively. |
| 表 2 不同培养条件下EGF、SCF、CSF1和LIF的mRNA相对表达量 Table 2 Relative mRNAlevels of EGF,LIF,SCF and CSF1 in human UCMSCs with different treatments |
ELISA结果显示(表 3),所有培养条件下胞浆提取物和上清均检测到SCF和CSF1,但不能检测到LIF(表 3中未列出),EGF仅在培养上清液中能检测到;添加视黄醇(1 μmol/L)后,培养上清和胞浆抽提液中SCF和CSF1含量均显著高于对照组(P<0.05),EGF含量差异不显著(P≥0.05)。总之,视黄醇(1 μmol/L)可显著提高人UCMSCs在体外培养时生成SCF和CSF1的能力。
| 表 3 不同培养条件下EGF、SCF和CSF1的蛋白表达量 Table 3 Protein expressions of EGF,SCF and CSF1 in the cells with different treatments |
诸多研究已证实UCMSCs具有骨髓和脂肪来源MSCs的生物学特性[20-21],同时也表达早期胚胎细胞和胚胎干细胞的多潜能性标志分子如Oct4、Nanog 和Sox2[22-24],免疫组化结果显示本实验分离所得的人UCMSCs与文献报道一致[22-23]。
人UCMSCs与骨髓来源MSCs具有相似的细胞因子表达谱,二者均表达EGF、SCF、LIF、M-CSF(CSF1)、IL-6、VEGF、Flt3配体(FL)和SDF,但也存在一些差异,如人UCMSCs 表达GM-CSF 和G-CSF,而骨髓来源MSCs却不表达[7, 9-10, 17, 25];另外,人UCMSCs的EGF表达量显著高于骨髓来源MSCs[9, 12]。本试验结果显示人UCMSCs转录表达EGF、SCF、CSF1 和LIF 的mRNA,结果与Hsieh等[7, 9]的报道一致;在蛋白质水平,ELISA结果显示细胞裂解液中无LIF表达,究其原因可能与所用LIF检测试剂盒灵敏度(只能检测浓度在70 ng/L以上)有关。
前已述及,SCF和CSF1是造血干细胞(HSCs)更新所必需的细胞因子,Doan等[22]报道EGF能刺激辐射损伤小鼠HSCs的再生恢复,而人UCMSCs体内外支持HSCs再生/增殖的能力亦早为诸多研究证实[1, 7]。本实验结果从mRNA转录和蛋白质水平均显示视黄醇(1 μmol/L)能显著提高SCF和CSF1的表达水平,进而可能增强人UCMSCs体外支持HSCs增殖的能力。Chen等[13]报道视黄醇(0.5 μmol/L)通过活化PI3K/Akt信号途径促进小鼠ESCs自我更新,而活化PI3K/Akt信号途径也促进MSCs产生促血管新生因子[6],因此视黄醇对人UCMSCs表达细胞因子的影响是否由PI3K/Akt信号途径活化所致,以及改变视黄醇浓度是否会引起其它相关因子表达谱改变,还需进一步研究确定。
综上所述,视黄醇(1 μmol/L)可促进体外培养人UCMSCs 的SCF 和CSF1 mRNA 转录和蛋白表达上调,进而为UCMSCs用于损伤修复、血管再生、支持造血等临床治疗提供理论依据。
| [1] | Fan CG, Zhang QJ, Zhou JR. Therapeutic potentials of mesenchymal stem cells derived from human umbilical cord[J]. Stem Cell Rev, 2011, 7 (1): 195-207. DOI: 10.1007/s12015-010-9168-8. |
| [2] | Sabapathy V, Sundaram B, Sreelakshmi VM, et al. Human wharton's jelly mesenchymal stem cells plasticity augments Scar-Free skin wound healing with hair growth[J]. PLoS One, 2014, 9 (4): e93726. DOI: 10.1371/journal.pone.0093726. |
| [3] | Dalous J, Larghero J, Baud O. Transplantation of umbilical cord-derived mesenchymal stem cells as a novel strategy to protect the central nervous system: technical aspects, preclinical studies, and clinical perspectives[J]. Pediatr Res, 2012, 71 (4, 2): 482-90. |
| [4] | Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine[J]. Exp Mol Med, 2013, 45 (11). |
| [5] | Bai LP, Li DT, Li J, et al. Bioactive molecules derived from umbilical cord mesenchymal stem cells[J]. Acta Histochem, 2016, 118 (8): 761-9. DOI: 10.1016/j.acthis.2016.09.006. |
| [6] | de Luca A, Gallo M, Aldinucci DA, et al. Role of the EGFR ligand/ receptor system in the secretion of angiogenic factors in mesenchymal stem cells[J]. J Cell Physiol, 2011, 226 (8): 2131-8. DOI: 10.1002/jcp.22548. |
| [7] | Hsieh JY, Wang HW, Chang SJ, et al. Mesenchymal stem cells from human umbilical cord Express preferentially secreted factors related to neuroprotection, neurogenesis, and angiogenesis[J]. PLoS One, 2013, 8 (8): e72604. DOI: 10.1371/journal.pone.0072604. |
| [8] | 何远东, 王玉, 彭江. 人脐带间充质干细胞中神经营养因子的表达[J]. 中华神经医学杂志, 2012, 11 (5): 438-42. |
| [9] | Lu LL, Liu YJ, Yang SG, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesissupportive function and other potentials[J]. Haematologica, 2006, 91 (8): 1017-26. |
| [10] | Hass R, Kasper C, B?hm S, et al. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC[J]. Cell Commun Signal, 2011, 9 (1): 12. DOI: 10.1186/1478-811X-9-12. |
| [11] | Khillan JS. Vitamin a/retinol and maintenance of pluripotency of stem cells[J]. Nutrients, 2014, 6 (3): 1209-22. DOI: 10.3390/nu6031209. |
| [12] | Chen LG, Khillan JS. Promotion of feeder-independent self-renewal of embryonic stem cells by retinol (vitamin A)[J]. Stem Cells, 2008, 26 (7): 1858-64. DOI: 10.1634/stemcells.2008-0050. |
| [13] | Chen LG, Khillan JS. A novel signaling by vitamin a/retinol promotes self renewal of mouse embryonic stem cells by activating PI3K/Akt signaling pathway via Insulin-Like growth factor-1 receptor[J]. Stem Cells, 2010, 28 (1): 57-63. |
| [14] | Rajala K, Vaajasaari H, Suuronen R, et al. Effects of the physiochemical culture environment on the stemness and pluripotency of human embryonic stem cells[J]. Stem Cell Stud, 2011, 1 (1): 3. DOI: 10.4081/scs.2011.e3. |
| [15] | Zhang SS, Sun JW, Pan SH, et al. Retinol (vitamin a) maintains Self-Renewal of pluripotent male germline stem cells (mGSCs) from adult mouse testis[J]. J Cell Biochem, 2011, 112 (4): 1009-21. DOI: 10.1002/jcb.v112.4. |
| [16] | 刘丹, 余树民, 刘欢欢, 等. 维生素A对体外培养人脐带间充质干细胞生长的影响[J]. 中国细胞生物学学报, 2014, 25 (6): 810-7. |
| [17] | 许文静, 侯克东, 袁玫, 等. 细胞因子和生长因子在人脐带Wharton胶间质干细胞的表达谱[J]. 中华创伤骨科杂志, 2013, 15 (12): 1071-5. |
| [18] | Ebata KT, Yeh JR, Zhang XF, et al. Soluble growth factors stimulate spermatogonial stem cell divisions that maintain a stem cell pool and produce progenitors in vitro[J]. Exp Cell Res, 2011, 317 (10): 1319-29. DOI: 10.1016/j.yexcr.2011.03.013. |
| [19] | Oatley JM, Oatley MJ, Avarbock MR, et al. Colony stimulating factor 1 is an extrinsic stimulator of mouse spermatogonial stem cell self-renewal[J]. Development, 2009, 136 (7): 1191-9. DOI: 10.1242/dev.032243. |
| [20] | Barberini DJ, Paiva Freitas NP, Maia L, et al. Equine mesenchymal stem cells from bone marrow, adipose tissue and umbilical cord: immunophenotypic characterization and differentiation potential[J]. Stem Cell Res Ther, 2014, 5 (1): 1. DOI: 10.1186/scrt390. |
| [21] | Amable PR, Telles Teixeira MV, Vieira Carias RB, et al. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton's jelly[J]. Stem Cell Res Ther, 2014, 5 (2): 1. |
| [22] | Hu L, Hu JQ, Zhao JJ, et al. Side-by-Side comparison of the biological characteristics of human umbilical cord and adipose tissue-derived mesenchymal stem cells[J]. Biomed Res Int, 2013 (9): 438243. |
| [23] | Jo CH, Kim OS, Park EY, et al. Fetal mesenchymal stem cells derived from human umbilical cord sustain primitive characteristics during extensive expansion[J]. Cell Tissue Res, 2008, 334 (3): 423-33. DOI: 10.1007/s00441-008-0696-3. |
| [24] | Lv FJ, Tuan RS, Cheung KM, et al. Concise review: the surface markers and identity of human mesenchymal stem cells[J]. Stem Cells, 2014, 32 (6): 1408-19. DOI: 10.1002/stem.1681. |
| [25] | Majumdar MK, Thiede MA, Haynesworth SE, et al. Human marrow-derived mesenchymal stem cells (MSCs) Express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages[J]. J Hematother Stem Cell Res, 2000, 9 (6): 841-8. DOI: 10.1089/152581600750062264. |
| [26] | Doan PL, Himburg HA, Helms KA, et al. Epidermal growth factor regulates hematopoietic regeneration after radiation injury[J]. Nat Med, 2013, 19 (3): 295-304. DOI: 10.1038/nm.3070. |
 2017, Vol. 37
2017, Vol. 37

