2. 广东三九脑科医院影像中心, 广东 广州 510510
2. Imaging Center, Guangdong 999 Brain Hospital, Guangzhou 510510, China
髓母细胞瘤是后颅窝常见的肿瘤,发病高峰在5~10岁,占儿童颅内肿瘤的25%~35%及成人颅内肿瘤的1% [1-2]。大多数儿童髓母细胞瘤在出现症状后6个月左右确诊,而复发的危险期多为首诊时的年龄加上9个月,即Collin法则[1, 3]。肿瘤单纯手术不易完全切除,术后联合放、化疗可以明显提高生存率,但对于术后化疗也只有46%的患者表现出完全缓解[4]。术前正确诊断及判断有无脑脊液种植转移对于手术或治疗方式的选择有重大的意义。目前在髓母细胞瘤诊断中,MRI传统序列能提供肿瘤的大小、边界、内部信号特征等形态学方面的特点,对比增强扫描可以反映血脑屏障的破坏,但其诊断价值有限,误诊率高[5-6]。弥散加权成像(diffusion weighted imaging, DWI)、氢质子波谱成像(1H-magnetic resonance spectroscopy, 1H-MRS)、三维动脉自旋标记(3D whole-brain arterial spin labeling, 3D ASL)能够无创的反映水分子的自由扩散、肿瘤的代谢物变化及血流灌注特点,对本病的诊断均有一定价值,但各自仍有不足之处。部分后颅窝肿瘤在DWI、ASL表现上存在重叠[7-8],而MRS对于良恶性肿瘤鉴别有一定意义,但单独使用MRS或联合ADC对于区分后颅窝肿瘤没有明显意义[9]。DWI、MRS及3D ASL综合起来便于从不同角度更为全面、透彻地了解肿瘤,有望从形态学和功能学层面对不典型髓母细胞瘤明确诊断。本文通过回顾性分析16例髓母细胞瘤的MRI图像,旨在探索DWI、MRS及3D ASL技术在髓母细胞瘤诊断及鉴别诊断中的价值,提高诊断准确率。
1 资料和方法 1.1 一般资料回顾性分析本院从2014年1月~2016年6月经手术病理证实、影像学资料完整(包含DWI、MRS或ASL)的髓母细胞瘤共16例。男10例,女6例,发病年龄≤10岁者10例,10~20岁4例,≥20岁者2例,儿童髓母细胞瘤年龄中位数为5岁,本组2例成人型髓母细胞瘤发病年龄均为26岁。临床表现主要有头痛、头晕、恶心呕吐、行走不稳等,病程3 d~1年,14例在1~3月之内。所有病例均行常规MRI平扫及增强扫描,5例行DWI检查,12例行MRS检查,5例行3D ASL检查。
1.2 检查方法采用GE Signa HDXT 3.0 T和Philips 1.5T磁共振扫描仪。常规序列包括轴位T1WI、T2WI、液体衰减反转恢复(FLAIR)序列和矢状位T1WI;MRI增强扫描造影剂使用Gd-DTPA,剂量为0.1 mmol/kg,速率为1.5~2.5 mL/s。
DWI:EPI SE序列,TR/TE=6000 ms/75.5 ms,b=0 s/mm2、1000 s/mm2。
1H-MRS:采用单体素方法测定。TR/TE=1500 ms/35 ms、144 ms,FOV=24 cm×24 cm,NEX=8;选择病灶中实性部分为感兴趣区,计算患侧氮-乙酰天门冬氨酸(NAA)、胆碱类化合物(Cho)、肌酸(Cr)的含量,计算Cho/Cr、NAA/Cr、Cho/NAA的比值。
3D ASL:FSE序列,TR/TE=4404 ms/9.8 ms,PLD=1525 ms,NEX=3。所得图像通过自带的Functool软件进行处理,自动生成伪彩图像,测定肿瘤灌注最高的区域及镜像侧的绝对血流量(CBF),相对血流量rCBF=肿瘤CBF/镜像侧CBF。
2 结果 2.1 病变部位、形态、大小15例单发,1例并发蚓部及双侧小脑半球多发肿块。9例位于蚓部-四脑室(图 1),5例发生于小脑半球(包括2例≥20岁的患者,图 2),1例发生于左侧桥小脑角区。10例呈圆形或类圆形,4例为浅分叶状,2例形态不规则。肿物最大径1~5.4 cm。
|
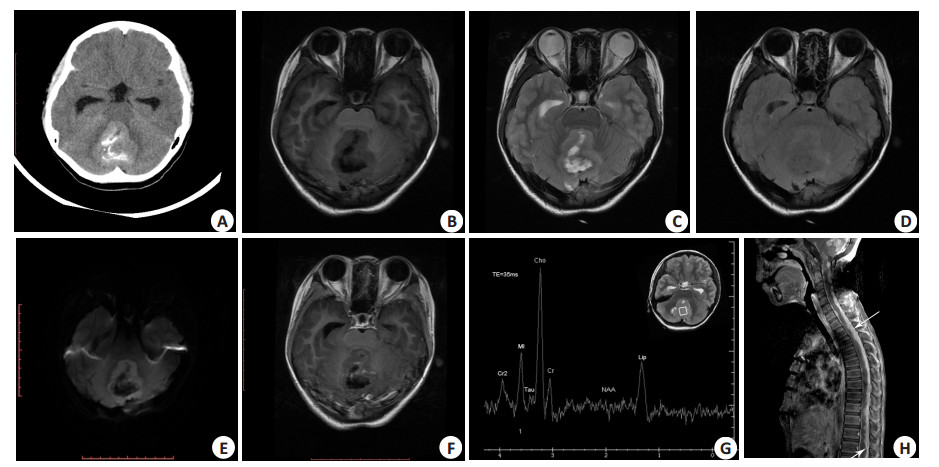
图 1 13岁,女,髓母细胞瘤,手术、放化疗后复发 Figure 1 A 13-year-old girl with medulloblastoma. A: CT images showing a mass in the cerebellar vermis. The mass is hyperattenuating on the noncontrast CT with patchy calcification; B-F: The tumor was slightly hypo-intense on T1WI, and was iso-intense or hyper-intense on T2WI and FLAIR with high signals on DWI. Low signal areas were identified representing cystic and necrotic foci in the lesion. Tumor showed mild patchy enhancement after contrast agent injection. G: 1H MRS examination showing significantly decreased NAA and Cr peak with a significantly increased Cho peak. An obvious taurine peak was noted at 3.4 ppm and an Lip peak at 1.3 ppm; H: Sagittal contrast-enhanced T1WI image at 8 months after radiotherapy and chemotherapy demonstrated strip enhancement along the spinal cord (arrows) suspicious for leptomeningeal metastases. |
|
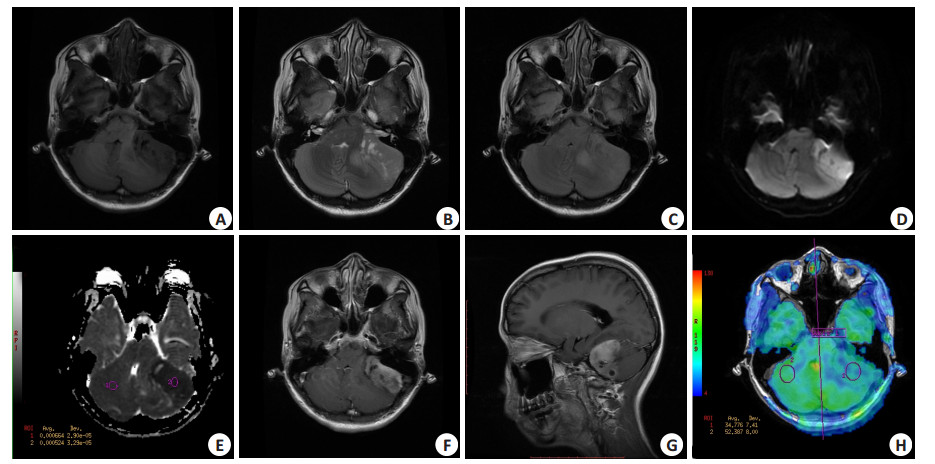
图 2 26岁,女,髓母细胞瘤,主诉行走不稳2月 Figure 2 Medulloblastoma in a 26-year-old female patient with walking instability for over 2 months. A-G: MRI showing a mass in the left cerebellar hemisphere with slight hypo-intense signals on T1WI and iso-intense or hyper-intense signals on T2WI and FLAIR. Small cystic and necrotic foci were detected. The lesions showed high signals on DWI and low signals on ADC with significant enhancement after contrast agent administration. H: 3D ASL showing hypoperfusion in the left hemisphere. |
T1WI上14例表现为稍低信号,2例呈等皮质信号。T2WI上8例呈等信号,8例呈稍高信号。FLAIR序列上10例呈等信号,6例呈稍高信号。1例可见团块状钙化,未见明显出血,12例可见长T1长T2囊变、坏死区。
2.3 增强扫描10例呈明显强化,6例呈斑片状或结节样轻度强化。
2.4 其他特征3例可见血管流空表现。8例病灶前方可见弧形脑脊液信号影。12例并发幕上脑室扩张积水。3例并发脑膜、脊膜转移。
2.5 DWI、MRS及ASL表现DWI序列中,5例病灶均呈高信号,ADC测值较对侧小脑组织明显降低(表 1);MRS示Cho峰均明显升高,NAA峰明显降低(4例未测及)。Cho/Cr比值显著增大3.5~15.6,Cho/NAA显著升高4.0~58,4例可见Lip峰,1例可见Lac峰,8例于3.4 ppm处紧邻胆碱峰之后可见Tau峰,呈轻度M型;5例髓母细胞瘤中3D ASL伪彩图均呈低灌注表现,其CBF值均低于对侧(表 2)。
| 表 1 病灶与对侧组织ADC值及DWI表现 Table 1 ADC value and DWI findings in the lesions and the contralateral tissue |
| 表 2 病灶与对侧组织CBF值及ASL伪彩图表现 Table 2 CBF values and ASL findings in the lesions and the contralateral tissue |
髓母细胞瘤是一种高度恶性的胚胎性肿瘤。目前多认为其来源于第四脑室顶部后髓帆神经上皮细胞的残余,其具有多向分化趋势,随着年龄的增加逐渐向上、向外迁移,因此儿童、成人髓母细胞瘤的好发部位存在差异[2]。随着对髓母细胞瘤的认识逐渐加深,2016版WHO中枢神经细胞肿瘤分类中根据基因表现将其分为WNT激活型、SHH激活型、非WNT/非SHH的Group3和4型,并且认为不同亚型有着较大的治疗和预后差异[10]。
儿童髓母细胞瘤多发生于小脑蚓部,而成人髓母细胞瘤常表现为四脑室、小脑半球或桥小脑角区占位[1, 6]。由于细胞密集、肿瘤含水分少,T1WI、T2WI及FLAIR序列上都倾向为等信号,增强后不同亚型表现为斑片状轻度~明显强化[1-2, 11]。髓母细胞瘤囊变多见,本组发生率为75%,可能与肿瘤生长速度快,而血液供应不足导致肿瘤组织缺血有关[12]。此外,也有学者提出囊变可能与肿瘤存在分泌功能有关[12]。髓母细胞瘤钙化少见,本组1例见钙化,与文献数据接近[6]。肿瘤常因压迫四脑室而导致幕上脑室梗阻、扩张,文献报道就诊时75%的患者已伴有脑积水[13]。髓母细胞瘤的常规MRI序列表现报道较多,但是误诊率高,对该病的定性诊断价值有限。DWI、MRS及3D ASL等序列能够无创地提供生理、生化信息,对病变的定性有重要价值。
DWI:通过施加扩散敏感梯度场来显示生物体中的水分子扩散,通过表观扩散系数(ADC)来表征水分子的扩散运动。目前DWI已经成熟地应用在胶质瘤分级诊断、淋巴瘤诊断及颅内囊性病变的鉴别中。恶性肿瘤由于细胞密度较高、细胞外间隙小,水分子弥散运动能力下降,组织的ADC值下降[14]。Rumboldt等[15]对后颅窝常见肿瘤的弥散进行研究,其结果示毛细胞星形细胞瘤的ADC值(1.65±0.27)显著的高于室管膜瘤(1.10±0.11)和髓母细胞瘤(0.66±0.15),且经方差分析三者之间有明显统计学差异。而邓小林等[16]结合ROC曲线定量分析ADC比值鉴别儿童常见后颅窝肿瘤,确定两个最佳鉴别阈值可将肿瘤ADC比值范围划分成3部分,ADC比值>1.87者为毛细胞星形细胞瘤,ADC比值 < 1.19者为髓母细胞瘤,ADC比值介于1.87与1.19之间为室管膜瘤,敏感度级特异度均达85%以上。本组患者DWI序列上均示弥散受限,ADC值明显低于对侧正常组织,与上述文献报道一致。单独DWI、ADC值测量不易区别高级别胶质瘤、转移瘤、脑膜瘤和淋巴瘤,但对于DWI高信号肿瘤ADC值可提供肿瘤内部组织学成分信息,对于后颅窝肿瘤的鉴别是一种简单、有效的方法[7]。
MRS:MRS可以无创定量地分析代谢物,目前在中枢神经系统肿瘤中主要应用于肿瘤与非肿瘤疾病的鉴别、治疗后评估等方面。Hollingworth等[17]回顾了MRS在颅内肿瘤中的应用,常规MRI脑肿瘤诊断准确率约55%,在此基础上加以MRS分析后可提高到71%。MRS中Cho水平的增高与细胞数量和细胞膜转换率有关,肿瘤恶性程度越高越明显。关于肿瘤分级的MRS研究中认为高级别胶质瘤Cho/Cr、Cho/NAA通常≥4 [18]。而髓母细胞瘤恶性增殖,Cho/NAA和Cho/Cr比率明显增高,比星形细胞瘤和室管膜瘤更为突出[12]。本组病例中Cho/Cr≥3.5和Cho/NAA≥4.0,提示肿瘤具有较高的侵袭性。本组4例中无法测及NAA峰,可能由于肿瘤细胞过于原始有关[19]。此外,部分研究在髄母细胞瘤中检测到氨基乙磺酸峰(Tau),并且认为其为髓母细胞瘤较为特征性的表现[19-20]。也有学者认为Tau并非髓母细胞瘤所特有,而与髓母细胞瘤的某种亚型的侵袭行为相关[21]。Tau峰位于3.3~3.4 ppm处,呈M型,TE=30 ms时位于基线之上,而当TE=140 ms时,Tau峰位于基线之下[19, 22]。本组病例中8例于Cho峰之后可见Tau峰,与文献报道一致。而且本组4例可见Lip峰,考虑多与肿瘤恶性增殖、坏死有关。MRS为颅内肿瘤提供了一种“仿真活检”技术,Cho/Cr和Cho/NAA的明显增高及较为特征性的Tau峰对髓母细胞瘤有提示作用,有助于提高诊断的准确性。
3D ASL:常规MRI对比增强反映的是肿瘤血脑屏障破坏的程度、并不能提供有关肿瘤血管生成的信息,而灌注成像可以反映病变新生血管的特征。3D ASL对动脉血进行磁标记,无需外源性对比剂即可获得灌注信息,优势明显。标记后延迟时间(post label delay, PLD)是从标记时刻到采集图像的时间,是ASL成像一个重要的参数,常用的有1025、1525、2525及3025 ms。老年人血流较慢,常选用长PLD。此外,血管性病变由于血流动力学改变多选用较长PLD,青年人、肿瘤性病变选用短PLD [8, 23]。本组均为儿童或青年人肿瘤患者,PLD选择1525 ms。恶性肿瘤细胞增生活跃,细胞内氧耗增加,诱导血管内皮生长因子生成,促进结构不成熟的肿瘤血管形成而使其灌注发生改变[24-25]。国内、外关于髓母细胞的灌注特点研究少,Warnke等[26]使用氙计算机体断层成像研究髓母细胞瘤的灌注特点,结果示肿瘤血流量和毛细血管通透性均较低,肿瘤区域血流量为19.86±6.8 mL/100 g/min,对侧正常小脑为45.46±12.03 mL/100 g/min,这也解释了为什么肿瘤部分切除后尽管行大剂量的化疗,病人的的反应率仍然十分有限[5, 26]。Theillac等[27]使用动态磁敏感增强扫描的方法,结果示髓母细胞瘤灌注较低(rCBF=1.19±0.39),并且经典型髓母细胞瘤rCBF < 2,而促纤维增生型髓母细胞瘤rCBF < 1,这可能与促纤维增生型髓母细胞瘤的纤维胶原基质较多有关。而Yeom等[28]使用ASL的方法,其结果示髓母细胞瘤的rCBF(2.87±1.74)显著高于毛细胞型星形细胞瘤(1.05±0.19),与室管膜瘤相比,髓母细胞瘤仍具有较高的rCBF,但两者之间存在部分重叠。李勇等[8]学者研究全脑ASL灌注在脑肿瘤分级中的应用,其中1例髓母细胞瘤ASL呈高灌注。而本组5例ASL中CBF值均降低,伪彩图呈低灌注。综合上述多项研究结果看来,髓母细胞瘤的rCBF范围较宽,推测可能预示着不同的治疗效果。也可能与不同的发病年龄、亚型之间血流灌注差异有关[28]。其次,不同的灌注方法及后处理方式的选择对结果也会有一定影响[29]。
综上所述,髓母细胞瘤细胞排列紧密,细胞间隙小,DWI呈明亮高信号;其恶性程度高,MRS上Cho常显著升高,Cr、NAA明显降低,此外,较为特征性的Tau峰也具有鉴别意义;髓母细胞瘤3D ASL常呈低灌注表现。上述特征结合常规MRI鉴别幕下常见的肿瘤如毛细胞星形细胞瘤、室管膜瘤不难。但发生于小脑半球、桥小脑角区的髓母细胞瘤仍需要与脑膜瘤、淋巴瘤和成人血管母细胞瘤鉴别。上述肿瘤在常规MRI及DWI、MRS及3D ASL表现上均有一定重叠,三者结合起来有助于对其明确诊断。(1)脑膜瘤:脑外肿瘤,广基底与脑膜相连,强化均匀,典型者可见“脑膜尾征”。脑膜瘤DWI亦呈高信号,ASL呈高灌注,MRS常表现为Cho峰显著升高,NAA峰的缺失和丙氨酸峰(Ala)的出现具有一定特征性;(2)淋巴瘤:好发于中老年男性,细胞排列紧密,常规序列呈偏等信号,多呈明显均匀强化。DWI呈高信号,且淋巴瘤为乏血供肿瘤,ASL表现为低灌注,但MRS中高耸的Lip峰是其较为特征性的表现;(3)血管母细胞瘤可发生于任何年龄,但主要见于30~40岁,多发生于幕下,典型者MRI表现呈“大囊小结节”型,结节T1WI呈低或高低混杂信号,T2WI上呈不均匀高信号,肿瘤内部及周围常出现增多、迂曲的流空血管影,增强后显著强化。血管母细胞瘤血管丰富,ASL实性结节多呈明显高灌注,但血管母细胞瘤DWI呈等或低信号。此外,其MRS也不具备肿瘤特征。
本研究病例数较少,且MRS缺乏长、短不同回波时间的扫描,对一些代谢物的分析可能存在误差。但DWI、MRS及3D ASL分别反映肿瘤水分子的自由运动、血流变化及代谢物的改变,便于更为全面、透彻的了解肿瘤。总而言之,对MRI常规序列不能定性或者疑诊髓母细胞瘤的患者,应建议行DWI、MRS、ASL等功能检查,了解本病形态学之外更多信息,有助于对不典型髓母细胞瘤明确诊断。
| [1] | Sarkar C, Pramanik P, Karak AK, et al. Are childhood and adult medulloblastomas different? A comparative study of clinicopathological features, proliferation index and apoptotic index[J]. J Neurooncol, 2002, 59 (1): 49-61. DOI: 10.1023/A:1016357731363. |
| [2] | Mittal P, Gupta K, Saggar K, et al. Adult medulloblastoma mimicking Lhermitte-Duclos disease: can diffusion weighted imaging help[J]. ? Neurol India, 2009, 57 (2): 203-5. DOI: 10.4103/0028-3886.51297. |
| [3] | Collins VP. Wilms' tumor: its behavior and prognosis[J]. J La State Med Soc, 1955, 107 (12): 474-80. |
| [4] | Rutkowski S, Bode U, Deinlein F, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone[J]. N Engl J Med, 2005, 352 (10): 978-86. DOI: 10.1056/NEJMoa042176. |
| [5] | 谭仲伦, 成官迅, 郭晓婷. 成人髓母细胞瘤MRI诊断及误诊分析[J]. 医学影像学杂志, 2015, 25 (6): 969-72. |
| [6] | 曹晶, 许乙凯, 余田, 等. 成人髓母细胞瘤的MRI诊断及鉴别诊断[J]. 南方医科大学学报, 2010, 30 (11): 2609-10. |
| [7] | 闫新成, 鱼博浪, 张明, 等. ADC值测量在颅脑DWI高信号肿瘤诊断中的价值[J]. 现代肿瘤医学, 2014, 22 (11): 2721-4. |
| [8] | 李勇, 乔飞, 孔祥泉, 等. 3D-ASL全脑灌注成像在脑肿瘤术前诊断与分级中的应用价值[J]. 临床放射学杂志, 2015, 34 (6): 871-5. |
| [9] | Schneider JF, Confort GS, Viola A, et al. Multiparametric differentiation of posterior fossa tumors in children using diffusionweighted imaging and short echo-time 1H-MR spectroscopy[J]. J Magn Reson Imaging, 2007, 26 (6): 1390-8. DOI: 10.1002/(ISSN)1522-2586. |
| [10] | Louis DN, Perry A, Reifenberger G, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary[J]. Acta Neuropathol, 2016, 131 (6): 803-20. DOI: 10.1007/s00401-016-1545-1. |
| [11] | Malheiros SM, Franco CM, Stavale JN, et al. Medulloblastoma in adults: a series from Brazil[J]. J Neurooncol, 2002, 60 (3): 247-53. DOI: 10.1023/A:1021178518361. |
| [12] | Fruehwald PJ, Puchner SB, Rossi A, et al. Magnetic resonance imaging spectrum of medulloblastoma[J]. Neuror-adiology, 2011, 53 (6): 387-96. DOI: 10.1007/s00234-010-0829-8. |
| [13] | Schubert MI, Wilke M, Muller-Weihrich S, et al. Diffusionweighted magnetic resonance imaging of treatment-associated changes in recurrent and residual medulloblastoma: preliminary observations in three children[J]. Acta Radiol, 2006, 47 (10): 1100-4. DOI: 10.1080/02841850600990300. |
| [14] | Kotsenas AL, Roth TC, Manness WK, et al. Abnormal diffusionweighted MRI in medulloblastoma: does it reflect small cell histology[J]. ? Pediatr Radiol, 1999, 29 (7): 524-6. DOI: 10.1007/s002470050636. |
| [15] | Rumboldt Z, Camacho D L, Lake D, et al. Apparent diffusion coefficients for differentiation of cerebellar tumors in children[J]. AJNR Am J Neuroradiol, 2006, 27 (6): 1362-9. |
| [16] | 邓小林, 文明, 吴晓凤, 等. ADC比值鉴别诊断儿童后颅窝肿瘤[J]. 中国医学影像技术, 2015, 31 (11): 1620-4. |
| [17] | Hollingworth W, Medina LS, Lenkinski RE, et al. A systematic literature review of magnetic resonance spectroscopy for the characterization of brain tumors[J]. AJNR Am J Neuroradiol, 2006, 27 (7): 1404-11. |
| [18] | 郭世萍, 孙静, 鱼博浪. 氢质子磁共振波谱在颅内肿瘤诊断中的应用[J]. 中国CT和MRI杂志, 2004, 2 (4): 50-3. |
| [19] | Tong Z, Yamaki T, Harada K, et al. In vivo quantification of the metabolites in normal brain and brain tumors by proton MR spectroscopy using water as an internal standard[J]. Magn Reson Imaging, 2004, 22 (7): 1017-24. DOI: 10.1016/j.mri.2004.02.007. |
| [20] | Kinoshita Y, Yokota A. Absolute concentrations of metabolites in human brain tumors using in vitro proton magnetic resonance spectroscopy[J]. NMR Biomed, 1997, 10 (1): 2-12. DOI: 10.1002/(ISSN)1099-1492. |
| [21] | Moreno A, Rey M, Montane JM, et al. 1H NMR spectroscopy of colon tumors and normal mucosal biopsies; elevated taurine levels and reduced polyethyleneglycol absorption in tumors may have diagnostic significance[J]. NMR Biomed, 1993, 6 (2): 111-8. DOI: 10.1002/(ISSN)1099-1492. |
| [22] | Panigrahy A, Krieger MD, Gonzalez-Gomez I, et al. Quantitative short echo time 1H-MR spectroscopy of untreated pediatric brain tumors: preoperative diagnosis and characterization[J]. AJNR Am J Neuroradiol, 2006, 27 (3): 560-72. |
| [23] | 胡英, 陈莉, 肖艳, 等. 正常人3D-ASL脑血流灌注最佳标记后延迟时间分析[J]. 中国医学影像技术, 2016, 32 (9): 1330-5. |
| [24] | Maia AJ, Malheiros SM, Da RA, et al. MR cerebral blood volume maps correlated with vascular endothelial growth factor expression and tumor grade in nonenhancing gliomas[J]. AJNR Am J Neuroradiol, 2005, 26 (4): 777-83. |
| [25] | Ginat DT, Mangla R, Yeaney G, et al. Correlation between dynamic contrast-enhanced perfusion MRI relative cerebral blood volume and vascular endothelial growth factor expression in meningiomas[J]. Acad Radiol, 2012, 19 (8): 986-90. DOI: 10.1016/j.acra.2012.04.006. |
| [26] | Warnke PC, Kopitzki K, Timmer J, et al. Capillary physiology of human medulloblastoma: impact on chemotherapy[J]. Cancer, 2006, 107 (9): 2223-7. DOI: 10.1002/(ISSN)1097-0142. |
| [27] | Theillac M, Meyronet D, Savatovsky J, et al. Dynamic susceptibility contrast perfusion imaging in biopsy-proved adult medulloblastoma[J]. J Neuroradiol, 2016, 43 (5): 317-24. DOI: 10.1016/j.neurad.2016.05.002. |
| [28] | Yeom KW, Mitchell L A, Lober RM, et al. Arterial spin-labeled perfusion of pediatric brain tumors[J]. AJNR Am J Neuroradiol, 2014, 35 (2): 395-401. DOI: 10.3174/ajnr.A3670. |
| [29] | Paulson ES, Schmainda KM. Comparison of dynamic susceptibility-weighted contrast-enhanced MR methods: recommendations for measuring relative cerebral blood volume in brain tumors[J]. Radiology, 2008, 249 (2): 601-13. DOI: 10.1148/radiol.2492071659. |
 2017, Vol. 37
2017, Vol. 37

