2. Department of Endocrinology and Metabolism, NanfangHospital, Southern MedicalUniversity, Guangzhou 510515,China
2. 南方医科大学南方医院内分泌与代谢科,广州广东510515
Cavernous hemangiomas are localized defects ofvascular morphogenesis caused by dysfunction occurring duringthe embryonic period.They representone type of multiple venous malformations characterized by benignlesions and are common duringinfancy andchildhood. Cavernous hemangiomas commonly involve the subcutaneous tissues and occur occasionally in thebrain or such visceral organsas the liver and adrenal glands. In 1860,the co-occurrenceof congenitalcutaneous and gastrointestinal hemangiomatosis wasnamed Blue rubberbleb nevus syndrome(BRBNS) byGascoyen. Over the last two centuries,only approximately 200 cases of BRBNS have been reported. The lesionscan appear throughoutthe whole body,andin some cases,BRBNS may co-occur with malignant or benign tumors[1-4].
The co-occurrence of cavernous hemangiomas and congenital heart disease was first reportedby Schneeweiss in 1982[5]. So far only 3 BRBNS patients with congenital heart disease have been reported[6-8]. In this report,we present a case of BRBNS complicated by ventricular septaldefect in a 15-year-old boy.
CASE REPORTThe 15-year-old boy presentedwith numerous masses onthe body trunk and extremities at birth and wasdiagnosed to have BRBNS. Surgery was performedon his back and right wrist at 6 monthsof age. He received subsequent Chinesemedicine and injectiontherapies at 4 years of age,and was treated intermittently in recent years with propranolol. Some of his lesions disappeared while new ones appearedduring his childhood. Surgical repair of his ventricular septaldefect (VSD) was performed when he was one year old. At 14 years of age,the boy was 160 cm tall,weighed 45 kg and was not active in physicalactivities. His stool appeared chocolate-colored on sporadicoccasions,fecal occult blood tests yielded positiveresults and urinalysis was normal. Laboratory analysesrevealed severe iron-deficiency anemia,which was confirmedby bone marrow biopsy.Laboratory analyses showedthat the patient had normal blood platelets with leukocytes of (2.65-5.14)×10-9/L,hemoglobin of 41-91 g/L,mean corpuscular volume of 62.8-81.2 fL,mean corpuscular hemoglobin of 15.7-17.7 pg,Fe of 1.7-20 μmol/L,transferrin saturation of 2.58-3.21 g/L and ferritin of 3.5-7.4 ng/mL.
Computed tomography (CT) of the chest showedinflammation on both sides of the lower lungs.Abdominal ultrasound revealeda hemangioma lesion inthe right adrenalgland,and the liver,gallbladder,pancreas and kidneys were normal. Capsule endoscopyshowed the presenceof multiple hemangiomas in theupper segment of the smallbowel. Gastroscopic and colonoscopic examinations revealed multiple vascular blebs in the gastric body and sigmoid colon (Fig. 1),andthe esophagus also contained hemangioma lesions. Magnetic resonanceimaging (MRI) of the brain showedthe presence of hemangioma lesionson both sides of the occipital lobe (Fig. 1) and in the parotid glands,facial subcutaneous tissue,nose and pharynx. Whole-body blood pool scintigraphy (WBBPS) using 99Tcm-labeledred blood cells(99Tcm-RBCs) by single-photon emission computed tomography (SPECT) displayed multipleradioactive foci in the subcutaneous tissues of the trunk, extremities,and other organs including the bilateralparotids and small intestine (Fig. 2).
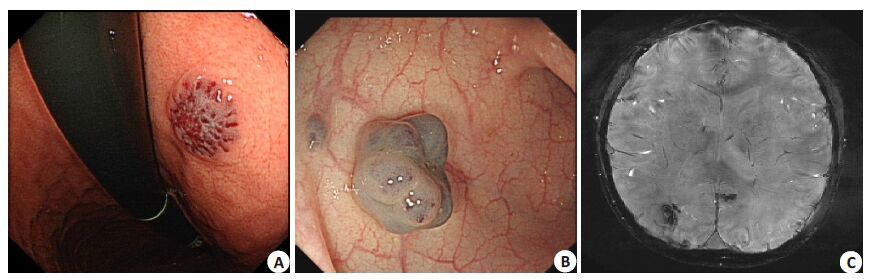
|
Figure 1 Gastroscopy revealedthe presence of a singlehemangioma lesion in the gastricbody (A). Colonoscopy revealed multiple varicosities in the sigmoid colon (B). Susceptibility-weighted imaging (SWI) clearly displayedmultiple draining veins onboth sides of the occipital lobe detected by their abnormally low signals (C). |
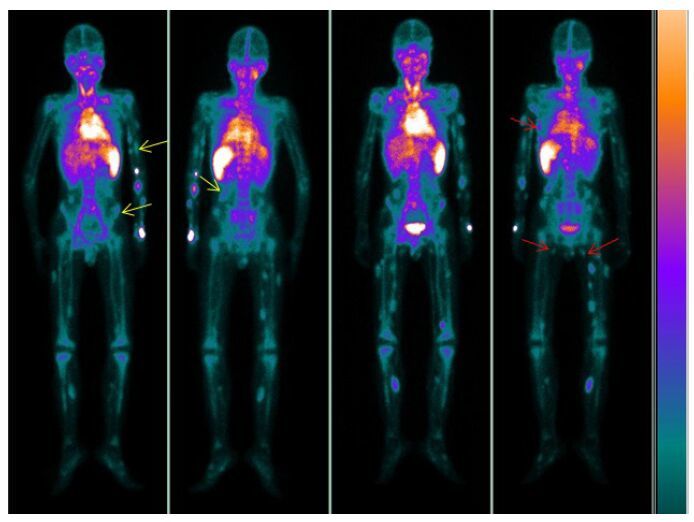
|
Figure 2 Whole-bodyblood pool scintigraphy of the patient.At 10 min,the images show extensive distribution of the radioactive tracer throughout the subcutaneous tissuesof the extremities,trunk and small intestine (yellow arrows). At 3 h,radionuclide pooling abnormally increasedas compared with that at 10 min (red arrows). |
Wouters et al demonstrated that hereditarycutaneomucosal venous malformation had an autosomal dominant inheritance pattern [9]. Patients with this disease may also have cardiac defects (cardiacmalformations). In our case,however,the patient'sfamily members do not presentwith similar conditions,so that whether his condition was hereditary in natureremains to be determined.
The patient was found to have multiple cavernous hemangiomas in the subcutaneous and mucosal tissues and in the cerebrum,nasopharynx,tongue,esophagus,gastric body,sigmoid colon and adrenal gland. Notably,the gastrointestinal lesions appearedto lead to relapse of intestinal bleeding. This boy weighedless than normal for his age with pale face and lips,indicating severeanemia; laboratory analysesrevealed that he had a lowhemoglobin level. His anemia improvedafter he was provided with Fe supplementation; however,the pain caused by the hemangioma lesions was aggravated.
Imaging studiesplay an important role in the diagnosis of BRBNS,which is a rare conditionand isdifficult to diagnose.Color Doppler is widely used todiagnose hemangioma and has a good sensitivity and specificity in detecting superficial hemangioma. MRIand CT are useful for detecting hemangiomas located in organs. 99Tcm-RBCs techniquehas been applied with WBBPS to diagnose congenitalvascular malformations,and has been shown to have a better sensitivity in detecting the lesions compared with MRI,angiography,and Doppler sonography[10-11]. We therefore recommend the use of combined imaging modalities in theexamination of hemangiomalesions.
| [1] | Kinner S, Herborn CU, Kroeger K. Simultaneous manifestation ofblue-rubber-bleb-nevus-syndrome and malignant melanoma[J]. Vasa,2006, 35 (4) : 239-41. DOI: 10.1024/0301-1526.35.4.239. |
| [2] | Nobuhara Y, Onoda N, Fukai K, et al. TIE2 gain-of-function mutationin a patient with pancreatic lymphangioma associated with bluerubber-bleb nevus syndrome: report of a case[J]. Surg Today,2006, 36 (3) : 283-6. DOI: 10.1007/s00595-005-3138-9. |
| [3] | Palleschi GM, Torchia D, Fabbri P. Blue rubber-bleb nevus syndrome:report of a case associated with osteoid osteomas[J]. J Dermatol,2005, 32 (7) : 589-93. DOI: 10.1111/jde.2005.32.issue-7. |
| [4] | Hoffman T, Chasko S, Safai B. Association of blue rubber bleb nevussyndrome with chronic lymphocytic leukemia and hypernephroma[J]. Johns Hopkins Med J,1978, 142 (3) : 91-4. |
| [5] | Schneeweiss A, Blieden L C, Shem-Tov A, et al. Coarctation of theaorta with congenital hemangioma of the face and neck and aneurysmor dilatation of a subclavian or innominate artery. A new syndrome[J]? Chest, 1982, 82(2): 186-7. |
| [6] | Bahl A, Raghavan A, Sinha S. Blue rubber bleb naevus syndrome andChiari malformation: high risk of peroperative haemorrhage[J]. TurkNeurosurg,2013, 23 (6) : 818-20. |
| [7] | Aroor S, Varma C, Mundkur SC. Blue rubber-bleb nevus syndromewhich was associated with an atrial septal defect: a case report[J]. JClin Diagn Res,2012, 6 (9) : 1566-7. |
| [8] | Giordano C, Battagliese A, di Gioia CR, et al. Blue rubber bleb nevussyndrome and pulmonary hypertension: an unusual association[J]. Cardiovasc Pathol,2004, 13 (6) : 317-22. DOI: 10.1016/j.carpath.2004.07.004. |
| [9] | Wouters V, Limaye N, Uebelhoer M, et al. Hereditary cutaneomucosalvenous malformations are caused by TIE2 mutations with widelyvariable hyper-phosphorylating effects[J]. Eur J Hum Genet,2010, 18 (4) : 414-20. DOI: 10.1038/ejhg.2009.193. |
| [10] | Kim YH, Choi JY, Kim YW, et al. Diagnosis and whole bodyscreening using blood pool scintigraphy for evaluating congenitalvascular malformations[J]. Ann Vasc Surg,2014, 28 (3) : 673-8. DOI: 10.1016/j.avsg.2013.02.025. |
| [11] | Das KJ, Sharma P, Naswa N, et al. Hybrid SPECT-CT with 99mTclabeledred blood cell in a case of blue rubber bleb nevus syndrome:added value over planar scintigraphy[J]. Diagn Interv Radiol,2013, 19 (1) : 41-3. |
 2016, Vol. 36
2016, Vol. 36
