2. Department of Reproductive Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China ;
3. Laboratory of Membrane Ion Channels and Medicine, Key Laboratory of Cognitive Science of State Ethnic Affairs Commission, College of Biomedical Engineering, South-Central University for Nationalities, Wuhan 430074, China ;
4. Department of Anesthesiology, Zhongnan Hospital of Wuhan University, Wuhan 430070, China
2. 生殖医学科,湖北 武汉 430030 ;
3. 中南民族大学生物医学工程学院国家民族事务委员会认知科学重点实验室,膜离子通道医学实验室,湖北 武汉 430074 ;
4. 武汉大学中南医院麻醉科,湖北 武汉 430064
Tactile allodynia is the most refractory symptom of neuropathic pain, which, along with hyperalgesia and spontaneous pain, seriously affects the quality of life of the patients [1, 2] As the factors associated with the onset and recovery of tactile allodynia are poorly understood [3, 4] conventional treatments have only limited effects with frequent undesirable side effects [5-7]. Novel therapeutic strategies are being explored, including gene knockdown and knock-in techniques and therapy targeting specific signal molecules, ion channels, transmitters, and receptors, and some of these strategies have achieved encouraging effects [8-12].
Evidence from gene-knockout mice shows that mechanical allodynia was transmitted almost exclusively by a specific subset of dorsal root ganglion (DRG) neurons that express vesicular glutamate transporter 3 (VGLUT3) [13], which highlights the importance of VGLUT3-positive neurons in the DRG as a promising target for relieving mechanical allodynia. But considering the wide distribution of VGLUT3 expression in the nervous system (as in the ventral cochlear nucleus, hippocampus, olfactory tubercle, and dorsal and medial raphe nuclei), the strategy of systemic Vglut3 gene knockout is associated with high risks of such adverse neurological effects as hearing loss and disorders in learning and memory [14-17]. In spite of the report of reliable analgesic effect of Vglut3 knockout in both inflammatory and neuropathic pain models [13], systemic Vglut3 knockdown does not appear to be feasible in clinical settings.
Cell type-specific RNA interference (RNAi) may provide an alternative approach to Vglut3 knock-down in the DRG. Several types of carriers, including viral vectors, have been tested for delivery of RNAi constructs to the DRG, and among these carriers, herpes simplex virus (HSV)-derived vectors have shown the potential as promising DRG-targeting vectors [18]. HSV possesses not only high tropism for peripheral sensory neurons but also the capacity to establish a life-long persistence in host cells (latent state of infection) in the intranuclear episomal form [19]. Natural uptake of HSV virion by the nerve terminal and its rapid retrogradeaxonal transport toward the nerve cell bodies offers a unique possibility of peripheral, noninvasive vector administration [20]. Recently, studies demonstrated that replication-deficient HSV type 1 (HSV-1) was capable of mediating the delivery of RNAi constructs targeting pain-related genes from the peripheral nerve to the DRG of mice in vivo, which resulted in highly effective and specific gene silencing in DRG neurons [21, 22]. Therefore, we hypothesize that selective knockdown of Vglut3 in the DRG by HSV-1-mediated RNAi can attenuate mechanical allodynia without causing systemic side effects.
In this study, we constructed a recombinant replication-defective HSV-1 vector carrying a short hairpin RNA (shRNA) that targeted Vglut3, and inoculated the vector in the sciatic nerve in a mouse model of mechanical allodynia (without heat allodynia) established by spared nerve injury (SNI) [23], and explored the efficiency of Vglut3 knockdown in the DRG and its analgesic effects in the mice.
MATERIAL AND METHODS Animal preparationslaboratory animal, Hunan, China) were kept at room temperature (24±1 ℃) on a 12 h light-dark cycle and given free access to laboratory chow and water. The experimental procedures and care of the animals were approved by the Animal Care and Use Committee of Tongji Hospital in line with the International Association of the Study of Pain (IASP) Guidelines for the Use of Animals in Research.
Nerve injury surgerySNI of the sciatic nerve was performed as previously described [23]. Briefly, the mice were an- esthetized with an intraperitoneal dose of ketamine (10 mg) and xylazine (0.5 mg/100 g body weight), and the left sciatic nerve and its 3 terminal branches were exposed cautiously. The common peroneal and the tibial nerves were tightly ligated with 5.0 silk, and a 2 to 4 mm segment distal to the ligation was removed. Touching or stretching the sural nerve was avoided to ensure its integrity. The muscle and skin were closed in turn. The mice receiving sham operation were subjected to exposure of the sciatic nerve and its branches without ligation or any injury to the nerve.
Construction of HSV-1 vectorsTwo recombinant HSV-1 vectors were constructed, namely HSV-1-U6-shvglut3-1 that contained red fluoresce protein gene (rfp) and the shRNA targeting Vglut3 (shvglut3-1), and HSV-1-U6-shvglut3-0 carrying rfp gene and a negative control shRNA sequence (shvglut3-0) that is not predicted to target any known vertebrate gene. Both of the viral vectors were under the control of a U6 promoter. According to the coding sequence of Vglut3 gene (GenBank Accession NM_ 182959.3), shvglut3-1 was designed (underlined sequence) and combined with a U6 promoter:
CAAGCTTAAG GTCGGGCAGG AAGAGGGCCT ATTTCCCATG ATTCCTTCAT ATTTGCATAT ACGATACAAG GCTGTTAGAG AGATAATTAG AATTAATTTG ACTGTAAACA CAAAGATATT AGTACAAAAT ACGTGACGTA GAAAGTAATA ATTTCTTGGG TAGTTTGCAG TTTTAAAATT ATGTTTTAAA ATGGACTATC ATATGCTTAC CGTAACTTGA AAGTATTTCG ATTTCTTGGC TTTATATATC TTGTGGAAAG GACGAAACAC CGGCGGTGGC TTCATTTCAA ACAACAGAGC TTTGTTTGAA ATGAAGCCAC CGTTTTTCTC GAGC.
Based on the established shRNA sequences against Vglut3, we disrupted its order and selected a sequence that shares no homology with Vglut3 gene as the control shRNA sequence (GCTGTTAGACCTATA GTAACC). Vglut3-0-shRNA (334 bp) with a U6 promoter was synthesized and verified by gene sequencing. Results of PCR identification showed that the synthesized plasmid contained a 334-bp sequence consistent with Vglut3- 0-shRNA. The sequence of U6-shvglut3-0 was otherwise identical with U6-shvglut3-1 except for the 271-335 fragments (CGGGCTGTTA GACCTATAGT AACCCAG AGC TGGTTACTAT AGGTCTAACA GCTTTTTCTC G AGA). Non-replicating HSV-1 vector backbone (Sino- GenoMax, Beijing, China) was constructed by deleting the virulent genes ICP27, ICP4, and ICP34.5 with genetic engineering methods. The plasmids pNX-U6- shvglut3-1 and pNX-U6-shvglut3-0 were obtained by inserting U6-shvglut3-1 and U6-shvglut3-0 sequences, respectively, to pNX (RFP) intermediate plasmid between Hind III and Xho I sites. Replication-defective HSV-1 vectors were generated by calcium phosphate co-transfection of complementing OG cells (Vero cell lines with stable expression of ICP27 and ICP4 proteins) with the intermediate plasmids described above and the HSV backbone. The genome structures were confirmed by PCR followed by sequencing. Transfection and interference effects of HSV-1-U6-shvglut3-0 (RFP) and HSV-1-U6-shvglut3-1 (RFP) were evaluated by detecting fluorescence expression and Western blotting. The positive viral plaques of HSV-1-U6-shvglut3-0 (RFP) and HSV-1-U6-shvglut3-1 (RFP) were subsequently amplified.
Transfection of OG cells in vitroOne day before transfection, cultured OG cells that stably expressed ICP4 and ICP27 proteins of HSV-1 (from Sino Geno Max Co., Ltd) were plated on a 6-well culture plate at the density of 1×105 to 1.5×105 cells in 2 mL DMEM supplemented with 10% culture medium to ensure a cell fusion rate of 90%-95% . The virus vector DNA of non-replicative HSV was co-transfected in OG cells with pNX-U6-Vglut3-1 (RFP) plasmid using calcium phosphate copreci-pitation method, and 3 to 6 days later, the cell sap was harvested when a lesionthe cell sap, several red CPE were picked for cloning to transfect OG cells again. The transfection was repeated several times until the virus was purified.
HSV-1 vector inoculation in micenamely the sham-operated group, SNI + normal saline (NS) group, SNI + HSV-1-U6-shvglut3-0 (HSV-1-NC) group and SNI+HSV-1-U6-shvglut3-1(HSV-1-shvglut3) group. Three days after SNI, the mice in HSV-1- shvglut3, HSV-1-NC, and NS groups were anesthetized with isoflurane and 5 μL of HSV-1-U6-shvglut3-1(1×108 pfu/mL), HSV-U6-shvglut3-0 (1 × 108 pfu/mL), and normal saline, respectively, were administered through a 10-μL microsyringe into the left sciatic nerve near the previous incision. The vectors were infused over a period of 5 min and the injection syringe was kept in place for an additional 1 min to allow for viral diffusion and absorption.
Assessment of tactile allodyniaThe mice were accommodated to the testing environment by exposure to the testing chambers for 20 min on 3 separate days prior to the preoperative testing. Behavioral tests of the mice were conducted on the day of SNI surgery (2 days before vector inoculation) and at 0, 4, 7, 14, 21 and 28 days after vector inoculation. The tests were performed at approximately the same time points ranging from 6:00 am to 6:00 pm each day [24]. All the tests were done by experimenters blinded to the surgery that the mice had received. Paw withdrawal threshold (PWT) was measured using the up-and-down testing paradigm with a series of von Frey filaments (0.008, 0.06, 0.1, 0.4, 0.6, 1.0, 1.4 and 2.0 g), which delivered approximately logarithmic incremental forces with the starting filament of 2 g [25]. Each filament was applied perpendicularly at the lateral side of the paw innervated by the sural nerve.
Assessment of heat hyperalgesiaHeat hyperalgesia was measured by Hargreaves' test using a plantar tester (model 7372, UGO BASILE, VA, Italy) [26]. Briefly, the mice were placed in individual plexiglass containers on a glass floor. After 30 min of acclimation, perpendicular radiant heat stimulation was applied through the glass floor to the lateral side of the planter surface of the hind paw, and paw withdrawal latencies were measured. The heat intensity was adjusted to produce a baseline latency of 10 s. A cut-off time of 20 s was applied to avoid tissue damage.
Detection of HSV-1 vector distribution after inoculationOn days 4, 7 and day 14 after HSV-1 inoculation, 2 mice from each group were anesthetized with isoflurane and decapitated. The spine of the mice was quickly separated using tissue scissors on ice. The L4/5 DRG and lumbar enlargement of the spinal cord were isolated, wrapped with tissue-freezing medium, rapidly transferred into the cryostat microtome, and sliced into sections 15 or 20 μm in thickness. The RFP fluorescence, which indicated the location of the HSV-1 vectors following retrograde axonal transport, was monitored with a fluorescent microscope (Zeiss Axiovert 100, Germany).
Western blot analysisThe L4/5 segment of the spinal cord was quickly removed at 0-4 ℃ and stored at -80 ℃ before use. The frozen tissues were homogenized in ice-cold RIPA lysis buffer containing 50 mmol/L Tris (pH 7.4), 150 mmol/L NaCl, 1% (V/V) Triton X-100, 1% sodium dexycholate, 1% SDS and complete, mini, EDTA-free protease inhibitor cocktail (Beyotime, China). Proteins were separated with 10% SDS-PAGE and then transferred to PVDF membranes (Bio-Rad) at 200 mA for 2 h. Nonspecific binding sites were blocked for 1 h with 0.1% (PBS-T) containing 5% nonfat milk at room temperature. The blots were incubated overnight at 4 ℃ with a primary antibody [mouse anti-VGLUT3 antibody (1:500, Abcam, USA) or mouse anti-β-actin antibody(1:400, Boster, China)]. The membranes were then incubated with the secondary antibody (HRP-conjugated goat anti-mouse, 1:10 000, Abcam, USA) for 1 h at room temperature. Images of the bands on the membranes were photographed and analyzed with a Licor odyssey scanner (Li-cor Biotechnology, USA). The relative expression of each protein was calculated as the ratio of signal density to β-actin density and was normalized by NS group.
ImmunohistochemistryTo distinguish fluorescent staining of the tissue from virus fluorescence, tissues were observed under a fluorescence microscope before staining to ensure full fading of the virus fluorescence. At 14 days after viral administration, the mice were anesthetized with ketamine (10 mg) and xylazine (0.5 mg/100 g) followed by transcardial perfusion with 20 mL physiological saline and then with 40 mL 4% paraformaldehyde (PFA) in PBS (0.1 mol/L, pH 7.4; 10 mL/min); PFA perfusion was carried out at a fast rate for the first 20 ml and then at a constant lowered rate. The L4/5 spinal cord and DRG were removed from the spine, fixed in 4% PFA overnight at 4 ℃, and then cryoprotected in a solution containing 30% sucrose in PBS (0.01 mol/L) for 24 h. The spinal cords and DRGs were sectioned in the coronal plane (20 μm and 10 μm in thickness, respectively) with a freezing microtome. After being rinsed in PBS for 3 times (5 min each time), the sections were permeabilized with 0.3% Triton X-100 in PBS for 30 min, incubated for 24 h at 4 ℃ with monoclonal mouse anti-VGLUT3 antibody (Abcam; 1:100 in PBS) with PBS as the negative control. After rinse with 0.1% PBS-T and deactivation of endogenous peroxidase with IgG antibody, and then immersed in SABC for 60 min. Subsequent DAB staining was conducted following the manufacturer's instructions (Boster, China). The expression of VGLUT3 protein was observed microscopically. The DRG sections were incubated with a fluorescent secondary antibody (goat-anti-mouse IgGCy3) for 2 h and the expression of VGLUT3 were examined under a Zeiss Axiovert 100 fluorescence microscope equipped with a Hamamatsu CCD digital camera.
Statistical AnalysisAll the data were presented as Mean ±SE and analyzed by one-way ANOVA followed by post-hoc Bonferroni test using SPSS 12.0 software. A P value less than 0.05 was considered to indicate a statistically significant difference.
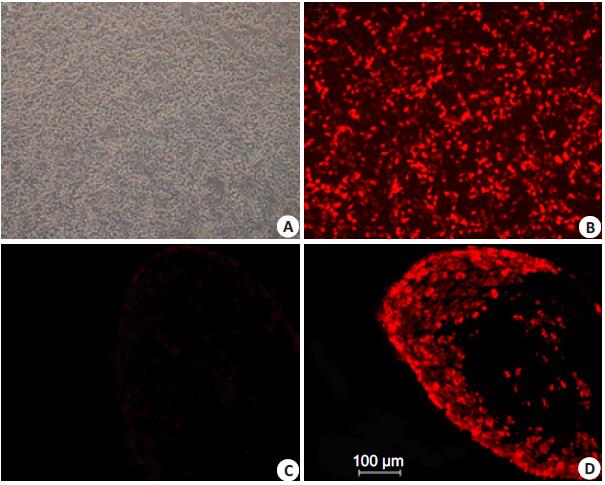
RESULTS Transfection of OG cells in vitro and tissue targeting effect of the virusRed fluorescent protein (RFP) gene was inserted at upstream of the two shRNA cassettes to facilitate purification of the vectors and verification of their DRG-targeting efficiency in vivo. The intermediate plasmid pNX-U6-shvglut3-1 or pNX-U6-shvglut3-0 was co-transfected with HSV-1 backbone in OG cells. The negative virus did not emit any fluorescence while the positive virus exhibited strong red fluorescence under a fluorescent microscope at 546 nm (Fig. 1). After purification and amplification, the reconstructed HSV-1 vectors were inoculated in the sciatic nerve in SNI mice. On days 4, 7 and 14 after the inoculation of the HSV-1 vectors, strong red fluorescence was detected in the L4/5 DRG (Fig. 1), but not in the spine or any other region of the nervous system. These results indicate that the reconstructed vectors possess DRG-targeting property.
|
Figure 1 OG cells transfected in vitro and DRG cells of the mice with HSV-1 vector inoculation. A: OG cells co-transfected with pNX-U6-shvglut3-0 and HSV-1 backbone; B: OG cells co-transfected with pNX-U6-shvglut3-1 and HSV-1 backbone; C: DRG cells of a mouse with saline injection in the sciatic nerve; D: DRG cells of a mouse with inoculation of the recombinant HSV-1 vector in the sciatic nerve following SNI. |
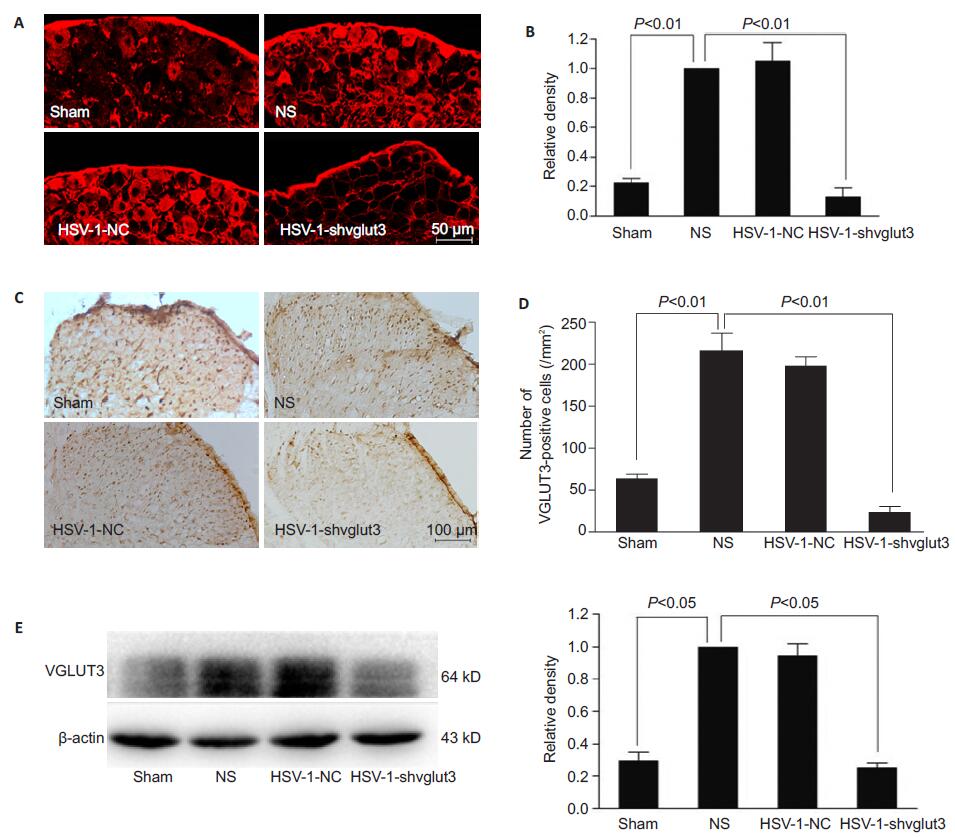
Immunofluorescence histochemistry revealed strong VGLUT3 protein expression after SNI in the DRG at L4/5 segments, which was fully reversed by inoculation of HSV-1-U6-shvglut3-1 in the sciatic nerve (Fig. 2). Immunohistochemistry detected similar change in the spinal dorsal horn at the L4/5 segments (Fig. 2). In NS group, the number of VGLUT3-positive cells increased significantly following SNI in the superficial zone of the ipsilateral spinal dorsal horn as compared with that in the sham-operated group (n=3, P˂0.01). Fourteen days after the viral administration, the number of VGLUT3-positive cells decreased obviously in HSV-1- shvglut3-1 group as compared with that in NS group (n= 3, P˂0.05) and was similar with that in the shamoperated group (n=3, P>0.05). No significant difference was found between HSV-1-NC and NS groups (n=3, P> 0.05). This down-regulation of VGLUT3 was further confirmed by Western blot analysis. Compared with that in sham-operated group, VGLUT3 expression increased significantly in the ipsilateral dorsal horn of the lumbar enlargement of the spinal cord in NS group (n=3, P˂ 0.05), and this SNI-induced up-regulation of VGLUT3 expression was fully reversed by HSV-1-U6- shvglut3-1 at 2 weeks after its inoculation (Fig. 2).
|
Figure 2 HSV-1-U6-shvglut3-1 inoculation in the sciatic nerve reverses SNI-induced enhancement of VGLUT3 expression in the DRG and spinal dorsal horn. A: Immunofluorescence histochemistry of VGLUT3 protein expression in the DRG at L4/5 segments in the 4 groups; B: Statistical analysis histogram for A; C: Immunohistochemistry for detecting VGLUT3 protein expression in the superficial zone of the ipsilateral spinal dorsal horn at L4/5 segments of the mice; D: Statistical analysis histogram for the result of immunohistochemistry; E: Western blot analysis of VGLUT3 expression. SNI-induced up-regulation of VGLUT3 expression was fully reversed by HSV-1- U6-shvglut3-1 at 2 weeks after sciatic nerve inoculation (One-way ANOVA with post hoc Bonferroni tests). |
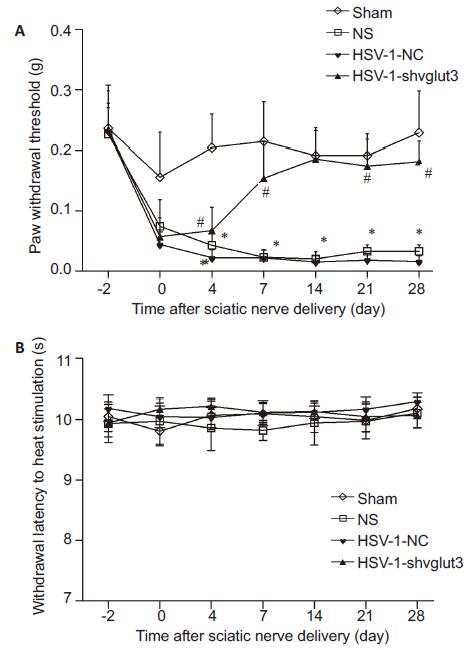
Throughout the study, no motor disturbance or other abnormal activities were found in any of the mice. SNI, but not sham surgery, produced significant tactile allodynia (Fig. 3). Inoculation of HSV-1-U6-shvglut3-1, but not HSV-1-U6-shvglut3-0, in the sciatic nerve significantly relieved tactile allodynia, and this effect persisted till the end of the observation period of 28 days. No obvious changes were observed in heat hypersensitivity after SNI or inoculation of HSV-1- U6-shvglut3-1.
DISCUSSIONIn this study, we found that the recombinant HSV-1 vector (HSV-1-U6-shvglut3-1) inoculated in the sciatic nerve effectively down-regulated VGLUT3 expression in the DRG neuron body and its central terminal in the superficial lamina of the spinal dorsal horn, and produced a strong and persistent analgesic effect in mice with mechanical allodynia induced by spared nerve injury.
|
Figure 3 Sciatic nerve inoculation of HSV-1-U6-shvglut3-1 reverses SNI-induced tactile allodynia in mice. Mechanical allodynia was measured with von Frey filaments using up and down testing paradigm. Paw withdraw threshold were expressed as Mean±SE (n=8). *P˂0.05 vs Sham group; #P˂0.01 vs NS and HSV-1-NC group (One-way ANOVA with post hoc Bonferroni test). |
For gene therapy for pain, researchers have tested natural pain-relieving molecules as the therapeutic targets that access endogenous antinociceptive circuitry, neurotransmitter receptors, or ion channels, whose expression changes in the pathological process of neuropathic pain [27-30]. But because of the wide distribution of neurotransmitters, receptors, and voltage-gated ion channels in the nervous system, it is difficult to selectively target pain-related pathways, even when using a DRG-targeting HSV vector [22]. As by mechanical hypersensitivity is transmitted exclusively VGLUT3- positive DRG neurons [13], Vglut3 serves as a promising target for knockdown. Vglut3 knockdown in the DRG by shRNA delivered by pseudo-latent recombinant HSV-1, which was transported through unmyelinated C fibers to the dorsal horn lamina I and II, resulted in reduced expression of VGLUT3 and produced a strong and persistent analgesic effect on mechanical allodynia 7 days after HSV-1 vector inoculation without observ- able side effects.
Consistent with a previous study using recombined HSV-1 to deliver interfering RNA to the DRG following its peripheral inoculation [21], we successfully delivered shRNA (HSV-1-U6-shvglut3-1) to the DRG after inoculation of the vector in the sciatic nerve. These peripherally inoculated non-replicating recombinant HSV-1 vectors reached the target DRG by retrograde axonal transport in a pseudo-latent state, and inhibited the expression of the target gene to modulate nociceptive neurotransmission from afferent nerve terminals to the dorsal horn of the spinal cord [22, 31, 32].
We did not observe any side effects of HSV-1 inoculation; in fact, studies suggest that herpes vectormediated gene transfer can alleviate pain without systemic side effects or inducing tolerance and can be used in combination with standard pain treatments [22]. Although we assessed the analgesic effects of HSV-1 vector inoculation within only 4 weeks, we assumed that the effect of such HSV vectors, in which transgene expression is driven by the promoter of immediate early gene of human cytomegalovirus, can persist for several weeks and can be reestablished by reinoculation of the vector [31].
In RNAi technique, nonspecific silencing may result from a non-sequence-specific effect caused by the virus, sequence-specific off-target effects, or induction of the interferon response. Our results showed that the negative control vector (HSV-1-U6-shvglut3-0) had no effect on pain threshold or target gene expression in the model mice, which could exclude any potential nonspecific effects induced by the HSV vector backbone.
This study was conducted to examine primarily the analgesic effect of HSV-1-shvglut3 on neuropathic pain. As VGLUT3-positive fibers were reported to contribute in a cause-dependent manner to the development of mechanical and cold hypersensitivity, further work is required to elucidate the mechanism underlying the analgesic effect of Vglut3 knockdown [33].
ConclusionOur results suggest that Vglut3 in the DRG is a promising therapeutic target for alleviating mechanical allodynia, and RNAi mediated by a HSV-1 vector inoculated in the peripheral nerves is a safe and efficient strateg.
| [1] | Luo F, Yang C, Chen Y, et al. Reversal of chronic inflammatory pain by acute inhibition of Ca2+/calmodulin-dependent protein kinase II[J]. J Pharmacol Exp Ther,2008, 325 (1) : 267-75. DOI: 10.1124/jpet.107.132167. |
| [2] | Hargreaves K, Dubner R, Brown F, et al. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia[J]. Pain,1988, 32 (1) : 77-88. DOI: 10.1016/0304-3959(88)90026-7. |
| [3] | Shirahama M, Ushio S, Egashira N, et al. Inhibition of Ca2+/ calmodulin-dependent protein kinase II reverses oxaliplatin-induced mechanical allodynia in rats[J]. Mol Pain,2012, 8 : 26. DOI: 10.1186/1744-8069-8-26. |
| [4] | Yeomans DC, Lu Y, Laurito CE, et al. Recombinant herpes vectormediated analgesia in a primate model of hyperalgesia[J]. Mol Ther,2006, 13 (3) : 589-97. DOI: 10.1016/j.ymthe.2005.08.023. |
| [5] | Moon JY, Song S, Yoon SY, et al. The differential effect of intrathecal Nav1.8 blockers on the induction and maintenance of capsaicin-and peripheral ischemia-induced mechanical allodynia and thermal hyperalgesia[J]. Anesth Analg,2012, 114 (1) : 215-23. DOI: 10.1213/ANE.0b013e318238002e. |
| [6] | Anesti AM, Peeters PJ, Royaux I, et al. Efficient delivery of RNAinterference to peripheral neurons in vivo using herpes simplex virus[J]. Nucleic Acids Res,2008, 36 (14) : e86. DOI: 10.1093/nar/gkn371. |
| [7] | Jones TL, Sweitzer SM, Wilson SP, et al. Afferent fiber-selective shift in opiate potency following targeted opioid receptor knockdown[J]. Pain,2003, 106 (3) : 365-71. DOI: 10.1016/j.pain.2003.08.006. |
| [8] | Wiffen PJ, Derry S, Moore RA, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia-an overview of Cochrane reviews[J]. Cochrane Database Syst Rev,2013 (11) : CD010567. |
| [9] | Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain[J]. Pain,2000, 87 (2) : 149-58. DOI: 10.1016/S0304-3959(00)00276-1. |
| [10] | Bourquin AF, Suveges M, Pertin M, et al. Assessment and analysis of mechanical allodynia-like behavior induced by spared nerve injury (SNI) in the mouse[J]. Pain,2006, 122 (1-2) : 14.e1-14. |
| [11] | Tzabazis AZ, Pirc G, Votta-Velis E, et al. Antihyperalgesic effect of a recombinant herpes virus encoding antisense for calcitonin generelated peptide[J]. Anesthesiology,2007, 106 (6) : 1196-203. DOI: 10.1097/01.anes.0000267603.32634.03. |
| [12] | Zhou Z, Peng X, Hao S, et al. HSV-mediated transfer of interleukin- 10 reduces inflammatory pain through modulation of membrane tumor necrosis factor alpha in spinal cord microglia[J]. Gene Ther,2008, 15 (3) : 183-90. DOI: 10.1038/sj.gt.3303054. |
| [13] | Poliani PL, Brok H, Furlan R, et al. Delivery to the central nervous system of a nonreplicative herpes simplex type 1 vector engineered with the interleukin 4 gene protects rhesus monkeys from hyperacute autoimmune encephalomyelitis[J]. Hum Gene Ther,2001, 12 (8) : 905-20. DOI: 10.1089/104303401750195872. |
| [14] | Jongen JL, Hans G, Benzon HT, et al. Neuropathic pain and pharmacological treatmen[J]. Pain Pract,2014, 14 (3) : 283-95. DOI: 10.1111/papr.2014.14.issue-3. |
| [15] | Gras C, Amilhon B, Lepicard EM, et al. The vesicular glutamate transporter VGLUT3 synergizes striatal acetylcholine tone[J]. Nat Neurosci,2008, 11 (3) : 292-300. DOI: 10.1038/nn2052. |
| [16] | Bennett DL, Boucher TJ, Armanini MP, et al. The glial cell linederived neurotrophic factor family receptor components are differentially regulated within sensory neurons after nerve injury[J]. J Neurosci,2000, 20 (1) : 427-37. |
| [17] | Liu J, Wolfe D, Hao S, et al. Peripherally delivered glutamic acid decarboxylase gene therapy for spinal cord injury pain[J]. Mol Ther,2004, 10 (1) : 57-66. DOI: 10.1016/j.ymthe.2004.04.017. |
| [18] | Watson ZL, Ertel MK, Lewin AS, et al. Adeno-associated virus vectors efficiently transduce mouse and rabbit sensory neurons coinfected with herpes simplex virus 1 following peripheral inoculation[J]. J Virol,2016, 90 (17) : 7894-901. DOI: 10.1128/JVI.01028-16. |
| [19] | Nicoll MP, Proenca JT, Efstathiou S. The molecular basis of herpes simplex virus latency[J]. FEMS Microbiol Rev,2012, 36 (3) : 684-705. DOI: 10.1111/j.1574-6976.2011.00320.x. |
| [20] | Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw[J]. J Neurosci Methods,1994, 53 : 55-63. DOI: 10.1016/0165-0270(94)90144-9. |
| [21] | Drew LJ, Macdermott AB. Neuroscience: unbearable lightness of touch[J]. Nature,2009, 462 (7273) : 580-1. DOI: 10.1038/462580a. |
| [22] | Seal RP, Wang X, Guan Y, et al. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors[J]. Nature,2009, 462 (7273) : 651-5. DOI: 10.1038/nature08505. |
| [23] | Saarto T, Wiffen PJ. Antidepressants for neuropathic pain[J]. Cochrane Database Syst Rev,2007 (4) : CD005454. |
| [24] | Elahi F, Callahan D, Greenlee J, et al. Pudendal entrapment neuropathy: a rare complication of pelvic radiation therapy[J]. Pain Physician,2013, 16 (6) : E793-7. |
| [25] | Ducic I, Felder JMR, Fantus SA. A systematic review of peripheral nerve interventional treatments for chronic headaches[J]. Ann Plast Surg,2014, 72 (4) : 439-45. DOI: 10.1097/SAP.0000000000000063. |
| [26] | Seal RP, Akil O, Yi E, et al. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3[J]. Neuron,2008, 57 (2) : 263-75. DOI: 10.1016/j.neuron.2007.11.032. |
| [27] | Dworkin RH. An overview of neuropathic pain: syndromes, symptoms, signs, and several mechanisms[J]. Clin J Pain,2002, 18 (6) : 343-9. DOI: 10.1097/00002508-200211000-00001. |
| [28] | Smith BH, Torrance N, Ferguson JA, et al. Towards a definition of refractory neuropathic pain for epidemiological research. An international Delphi survey of experts[J]. BMC Neurol,2012, 12 : 29. DOI: 10.1186/1471-2377-12-29. |
| [29] | Molet J, Pohl M. Gene-based approaches in pain research and exploration of new therapeutic targets and strategies[J]. Eur J Pharmacol,2013, 716 (1-3) : 129-41. DOI: 10.1016/j.ejphar.2013.01.073. |
| [30] | Glorioso JC, Fink DJ. Herpes vector-mediated gene transfer in the treatment of chronic pain[J]. Mol Ther,2009, 17 (1) : 13-8. DOI: 10.1038/mt.2008.213. |
| [31] | Cheng XR, Yang Y, Zhou WX, et al. Expression of VGLUTs contributes to degeneration and acquisition of learning and memory[J]. Neurobiol Learn Mem,2011, 95 (3) : 361-75. DOI: 10.1016/j.nlm.2011.01.010. |
| [32] | Ruel J, Emery S, Nouvian R, et al. Impairment of SLC17A8 encoding vesicular glutamate transporter-3, VGLUT3, underlies nonsyndromic deafness DFNA25 and inner hair cell dysfunction in null mice[J]. Am J Hum Genet,2008, 83 (2) : 278-92. DOI: 10.1016/j.ajhg.2008.07.008. |
| [33] | Dogrul A, Gardell LR, Ossipov MH, et al. Reversal of experimental neuropathic pain by T-type calcium channel blockers[J]. Pain,2003, 105 (1-2) : 159-68. |
 2016, Vol. 36
2016, Vol. 36
