2. 中国医科大学公共卫生学院环境卫生教研室, 辽宁沈阳 110001
2. Department of Environmental Health, College of Public Health, China Medical University, Shenyang 110001, China
虽然有关心肌梗死的药物治疗(抗血小板药物,ACEI类药物,调脂类药物)及侵入性治疗(PCI及CABG)已取得了显著进展,但是心肌梗死后心衰的死亡率和发病率仍然居高不下。原因在于目前的治疗措施并不能完全挽救已经梗死的心肌,心肌梗死后会形成较大的瘢痕组织,造成左室心肌重构,从而造成严重的左室功能不全。鉴于此,需要探索能够抑制心肌梗死后左室重构,再生心肌细胞的治疗措施,从而逆转心衰患者的不良预后。
目前有研究提示可注入型生物材料能够改善梗死心肌的左室重构,成为治疗急性心肌梗死研究的热点之一[1]。Dai等[2]通过研究证实向大鼠心肌梗死心肌内注入心肌组织源性的脱细胞化细胞外基质(ECM)能够增加心肌梗死后的左室壁厚度,防止左室矛盾性收缩膨出,改善心肌梗死后的左室射血分数(LVEF)。
也有研究提示心肌球源性心肌干细胞(CDCs)能够自我更新,向心肌细胞或血管细胞分化[3],促进鼠心肌梗死后的心肌细胞再生并且改善心功能[4]。同种异体CDCs治疗大鼠心肌梗死安全有效,能够促进心肌再生,改善心脏功能[5]。有研究表明向猪的梗死心肌组织内移植同种异体CDCs治疗心肌梗死,能够减轻左室重构,改善心肌整体和局部功能,减低心肌梗死瘢痕面积,并且还能够增加存活心肌组织量[6],心衰病人的CDCs也具有同样的治疗效果[7]。
然而,移植到宿主心肌内干细胞的分化率非常有限[8-9],从而影响干细胞治疗心肌梗死的后续疗效;有研究亦表明可注入型天然生物材料可用作移植干细胞载体从而增加移植干细胞的分化率和存活率[10-13]。基于上述研究经验[14-16],我们推测CDCs联合可注入型细胞载体注入梗死心肌能够促进CDCs增殖,分化;增加心肌梗死左室壁厚度,降低心肌梗死面积,改善左室功能,从而为今后心肌梗死及心衰的临床治疗提供理论依据。
1 材料和方法本动物实验得到了辽宁省实验动物管理委员会的同意并严格按照2011年国家卫生部关于“实验动物使用及保护指南”进行实验动物操作。
1.1 材料IMDM培养液,胎牛血清,L-谷氨酸,2-巯基乙醇,胰蛋白酶,不含钙镁的磷酸盐缓冲液(PBS),纤维连接蛋白,多聚-D-赖氨酸,10×MEM;兔抗α-SMA多克隆抗体,兔抗vWF多克隆抗体,小鼠抗α-SA单克隆抗体(Abcam公司);兔抗c-kit多克隆抗体,FITC-标记二抗,PE-标记二抗(SantaCruz公司);Ⅰ型鼠尾胶原胶(3.75 mg/mL)(碧云天);T10 basic ULTRA-TURRAX匀浆机(IKA, Wilmington, North Carolina),FACSCAN流式细胞仪(美国Becton Dickison公司)。
1.2 方法 1.2.1 心肌细胞外基质(ECM)的制备及检测按照Dai等[6]方案制备大鼠心肌源性ECM。具体方案如下:取雌性Wistar大鼠心脏,组织粉碎机(IKA, Wilmington, NorthCarolina)将心肌组织做成匀浆悬液,离心去上清。应用DNAse和RNAse去除核酸物质。通过反复离心洗涤最终将得到的ECM置于-80 ℃保存备用。体质量200 g左右的大鼠心脏可制备10 mg的ECM。
Ⅰ型鼠尾胶原胶(3.75 mg/mL)3 mL+10×MEM 1 mL+nuclease free无菌水6 mL,配置成10 mL浓度为1 mg/mL的Ⅰ型鼠尾胶原胶溶液,制备成1 mg/mL的ECM溶液,可用于包被培养皿。HE染色检测ECM中是否有细胞成分残余。
1.2.2 大鼠CDC分离及培养成年Wistar雄性大鼠,体质量170±10 g。按照Smith等[4]CDC分离及培养方法进行,无菌条件下取Wistar大鼠心脏,PBS彻底清洗,将心肌组织剪碎成大约1 mm3的组织块,采用酶消化法以CEM培养液(IMDM;20% FBS;1 U/mL青链霉素,0.2 mmol/L L-谷氨酸,0.1 mmol/L 2-巯基乙醇)培养组织块源性细胞(EDC)。1~2周后当EDCs生长达到70%~80%融合时,采集EDCs。将EDC以50 000~100 000/孔的密度移入12孔培养板中加入CGM培养液(35% IMDM/65% DMEM/F-12混合液,3.5% FBS,1%青霉素-链霉素,1% L-谷氨酸,0.1 mmol/L 2-巯基乙醇,1单位/mL凝血酶,2% B-27溶液,80 ng/mL bFGF,25 ng/mL EGF以及4 ng/mL心肌营养素-1)培养心肌球(CS)。6~7 d后心肌球生成,采集心肌球重新平铺于FN包被培养皿中继续培养获得CDC“。心肌球”平铺6~7 d后,采集并鉴定CDCs(3代内的CDCs)。
1.2.3 大鼠CDC体外荧光鉴定c-kit表达及CDC移植前体外标记传代2代的CDC移植前进行免疫荧光检测其c-kit表达情况,应用Gibco公司的CM-Dil标记CDC细胞膜,CDC显示红荧光。c-kit采用FITC标记,显示绿荧光,若CDC表达c-kit,则显示为黄荧光(红绿结合显示黄色)。具体操作步骤按照Gibco公司的CM-Dil标记活细胞方案进行。
1.2.4 心肌梗死模型的建立,CDC移植及心肌梗死后心功能评估8周龄雌性wistar大鼠通过开胸永久性结扎前降支的方法建立大鼠心肌梗死模型。心肌梗死模型建立后,心肌梗死大鼠分为4组:(1)对照组:单独150 μL IMDM注入组(n=6,Ⅰ组);(2)150 μL ECM组(n=6,E组);(3)106CDC/150 μL IMDM组(n=6,IC组);(4)106CDC/150 μL ECM组(n=6,EC组);上述4组均采用心肌梗死周边4点注入法注入心肌梗死周边。所有实验大鼠在心肌梗死模型建立前及心肌梗死后3周,采用小动物心彩超(GE Vivid 7 Dimension ultrasound apparatus, M12S PRO, probe frequency range of 4~12 MHz)。测量左室后壁厚度(LVPW),左室舒末容积(EDV),左室缩末容积(ESV),左室射血分数(LVEF),左室舒末内径(LVIDd),左室缩末内径(LVIDs),左室缩短分数(LVFS)。
1.2.5 心肌组织学分析各组心肌梗死后3周,10%水合氯醛麻醉各组大鼠,取大鼠心脏,OCT包埋,制作冰冻切片,行Masson染色,应用Image Pro Plus 5.0(Media Cybernetics Inc, Bethesda, Md)测量心肌梗死阳性面积百分比。心肌组织冰冻切片行组织切片免疫荧光染色鉴定在体CDC表达von Willebr-and factor(vWF),alpha smooth muscle actin(α-SMA),alpha sarcom-eric actin(α-SA)情况,CDC移植前应用Gibco公司的CM-Dil标记CDC细胞膜,CDC显示红荧光。采用FITC标记,显示绿荧光,若CDC表达vWF,α-SMA或α-SA,则显示为黄荧光,细胞核应用DAPI染色呈蓝色。测量阳性面积百分比及积光光密度OD(IC组vs EC组)。
1.2.6 统计学分析实验所得数据以平均值±标准差表示,应用SPSS 13.0软件单因素方差分析(One Way ANOVA)方法进行多组间差异的显著性检验,进一步两组间比较采用LSD-t检验(Fisher's least significant difference t-test),以P < 0.05为差异有统计学意义。
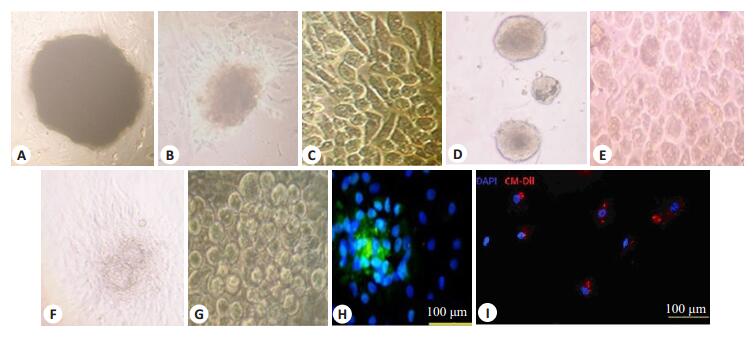
2 结果 2.1 大鼠CDC分离及培养,鉴定,标记以及大鼠心肌ECM制备后HE染色心肌组织块平铺FN包被的培养皿3~5 d后,从心肌组织块中生长出EDC,包括纤维细胞样和在倒置显微镜下观察为小圆亮2种形态细胞(图 1A~C)。CDC平铺于PDL包被的12孔培养板后5~7 d可生长出一代心肌球(图 1D,E),采集一代心肌球平铺于FN包被的60 mm培养皿后3~5 d生长出CDC(图 1F,G)。体外免疫荧光检测提示心肌球中心部为大量表达c-kit的细胞,心肌球平铺后的CDC也表达c-kit(图 1H)。
|
图 1 心肌球源性心肌干细胞体外培养形态学表现 Figure 1 Morphology of cardiosphere-derived cells (CDC) cultured in vitro (C, G, H, I: original magnification: ×200; A, B, D, E, F: ×100). |
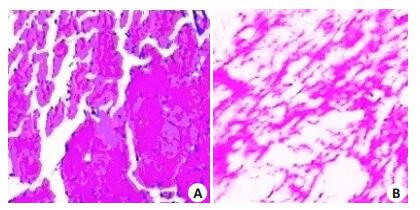
本实验中所应用ECM的制备方法可彻底清除细胞成分,并完整的保留了ECM的成分及结构(图 2)。
|
图 2 大鼠心肌ECM光镜表现 Figure 2 HE Staining of rat heart-derived extracellular matrix(×100). A: Normal control; B: ECM. |
心肌梗死造模前(E组,EC组,Ⅰ组及IC组)IVS,EDV,ESV,LVEF,LVIDd,LVIDs及FS%均无显著性差异,如表 1所示。心肌梗死3周后,与其他3组相比较(EC vs E vs IC vs Ⅰ),EC组的LVPW、EDV、ESV、LVEF、LVIDd、LVIDs、FS%显著性差异(P < 0.05,表 2);E组与IC组各项指标无统计学差异(表 2)。
| 表 1 心肌梗死前心脏彩超测量指标 Table 1 Parameters measured by cardiac ultrasonography before myocardial |
| 表 2 心肌梗死3周后心脏彩超测量指标 Table 2 Parameters measured by cardiac ultrasonography 3 weeks after myocardial infarction(n=6, Mean±SD) |
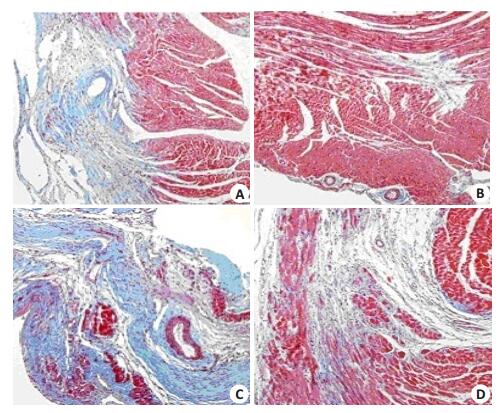
心肌梗死3周后,各组(E组,EC组,I组,IC组)大鼠心脏标本行Masson染色,与其他各组比较,EC组的心肌梗死纤维化阳性面积比Aa(%)最低,差异有统计学意义,E组与IC组心肌梗死纤维化阳性面积比Aa(%)无统计学差异(P > 0.05,图 3,表 3)。
|
图 3 心肌梗死3周后,各组Masson染色结果 Figure 3 Masson staining 3 weeks after myocardial infarction (×100). A: Group E; B: Group EC; C: Group I; D: Group IC. |
| 表 3 大鼠心肌梗死3周后,心肌Masson染色及荧光结果 Table 3 Results of Masson staining and immunostaining 3 weeks after myocardial infarction (n=6) |
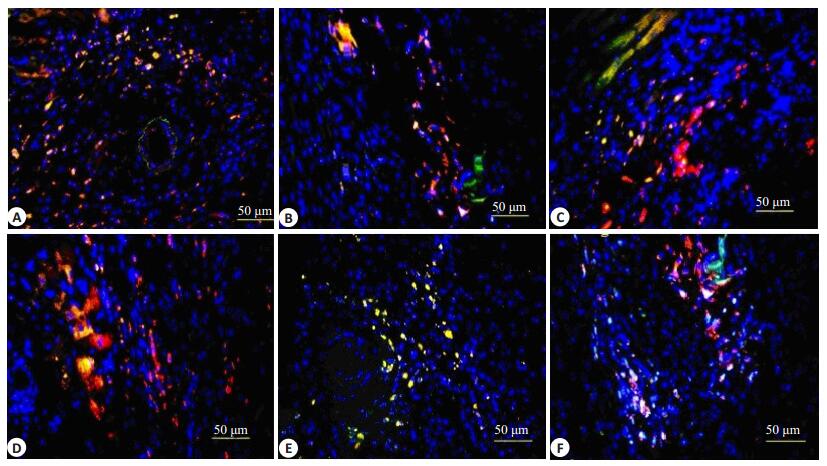
心肌梗死后3周,EC组v-WF、α-SA以及α-SMA表达阳性面积比,即表达黄色荧光面积比较IC组显著升高(P < 0.05,图 4,表 3)。
|
图 4 心肌梗死3周后各组免疫荧光表现 Figure 4 Immunofluorescence staining 3 weeks after myocardial infarction. A: v-WF in Group EC; B: v-WF in Group IC; C: α-SA in Group EC; D: α-SA in Group IC; E: α-SMA in Group EC; F: α-SMA in Group IC. |
与前述研究结果相似,本实验细胞培养法获得的表达c-kit的CDC细胞,具有自我复制及多项分化潜能[17]。目前培养及鉴定CDCs的技术已经成熟[18-24],因而可较容易获得CDCs且CDC治疗缺血性心肌病及心肌梗死的疗效确实肯定。
近年来有研究报道可注入型生物材料可用作干细胞移植载体从而提高干细胞体内移植的定植率及存活率[10-12],改善心肌梗死治疗效果。
我们在此次研究中采用ECM联合心肌球源性心肌干细胞直接心肌内注入法治疗大鼠急性心肌梗死。结果提示:与单独CDC移植组相比较,以心肌组织源性的细胞外基质作为CDC移植载体能够促进心肌梗死后3周CDC向心肌细胞(α-SA),血管平滑肌细胞(α-SMA)以及血管内皮细胞(v-WF)分化(图 4)。可能机制为本实验ECM的提取方法较好的保留了心室肌细胞外基质的成分及结构(图 2),为移植后的CDC提供了与原心脏相似的微环境,从而促进后续的心肌梗死后心功能(表 1,2)以及心脏结构(表 1~3)的改善。与先前研究结果相似[2],我们的研究提示单独ECM组(E组)与单独CDC组(IC组)两组心肌梗死后心脏结构及心脏功能的治疗效果相似。可能机制为间接的旁分泌机制以及直接的心肌再生机制,包括移植干细胞及内源性心肌干细胞分化为心肌,血管细胞[2]。
以同种异体心肌干细胞治疗急性心肌梗死作为研究内容的两项临床研究(ALLSTAR, CAREMI)正在进行当中,并且初期临床结果是令人鼓舞的。基于本研究中心肌组织源性的ECM制备过程的简单可行以及其独立治疗效果不逊于单独CDC治疗效果,我们有理由相信联合ECM及CDC治疗急性心肌梗死将有广阔的临床应用前景。
| [1] | Li Z, Fan Z, Xu Y, et al. pH-Sensitive and thermosensitive hydrogels as Stem-Cell carriers for cardiac therapy[J]. ACS Appl Mater Interfaces,2016, 8 (17) : 10752-60. DOI: 10.1021/acsami.6b01374. |
| [2] | Dai W, Gerczuk P, Zhang Y, et al. Intramyocardial injection of heart tissue-derived extracellular matrix improves postinfarction cardiac function in rats[J]. J Cardiovasc Pharmacol Ther,2013, 18 (3) : 270-9. DOI: 10.1177/1074248412472257. |
| [3] | Sun Y, Chi D, Tan M, et al. Cadaveric cardiosphere-derived cells can maintain regenerative capacity and improve the heart function of cardiomyopathy[J]. Cell Cycle,2016, 15 (9) : 1248-56. DOI: 10.1080/15384101.2016.1160973. |
| [4] | Smith RR, Barile L, Cho HC, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens[J]. Circulation,2007, 115 (7) : 896-908. DOI: 10.1161/CIRCULATIONAHA.106.655209. |
| [5] | Malliaras K, Li TS, Luthringer D, et al. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells[J]. Circulation,2012, 125 (1) : 100-12. DOI: 10.1161/CIRCULATIONAHA.111.042598. |
| [6] | Tseliou E, Kanazawa H, Dawkins J, et al. Widespread myocardial delivery of Heart-Derived stem cells by nonocclusive Triple-Vessel intracoronary infusion in porcine ischemic cardiomyopathy:superior attenuation of adverse remodeling documented by magnetic resonance imaging and histology[J]. PLoS One,2016, 11 (1) : e0144523. DOI: 10.1371/journal.pone.0144523. |
| [7] | Cheng K, Malliaras K, Smith RR, et al. Human cardiospherederived cells from advanced heart failure patients exhibit augmented functional potency in myocardial repair[J]. JACC Heart Fail,2014, 2 (1) : 49-61. DOI: 10.1016/j.jchf.2013.08.008. |
| [8] | Dow J, Simkhovich BZ, Kedes L, et al. Washout of transplanted cells from the heart:a potential new hurdle for cell transplantation therapy[J]. Cardiovasc Res,2005, 67 (2) : 301-7. DOI: 10.1016/j.cardiores.2005.04.011. |
| [9] | Cheng K, Li TS, Malliaras K, et al. Magnetic targeting enhances engraftment and functional benefit of iron-labeled cardiospherederived cells in myocardial infarction[J]. Circ Res,2010, 106 (10) : 1570-81. DOI: 10.1161/CIRCRESAHA.109.212589. |
| [10] | Dai W, Hale SL, Kay GL, et al. Delivering stem cells to the heart in a collagen matrix reduces relocation of cells to other organs as assessed by nanoparticle technology[J]. Regen Med,2009, 4 (3) : 387-95. DOI: 10.2217/rme.09.2. |
| [11] | Wang H, Zhou J, Liu Z, et al. Injectable cardiac tissue engineering forthe treatment of myocardial infarction[J]. J Cell Mol Med,2010, 14 (5) : 1044-55. |
| [12] | Cheng K, Shen D, Smith J, et al. Transplantation of platelet gel spiked with cardiosphere-derived cells boosts structural and functional benefits relative to gel transplantation alone in rats with myocardial infarction[J]. Biomaterials,2012, 33 (10) : 2872-9. DOI: 10.1016/j.biomaterials.2011.12.040. |
| [13] | Hale SL, Dai W, Dow JS, et al. Mesenchymal stem cell administration at coronary artery reperfusion in the rat by two delivery routes:a quantitative assessment[J]. Life Sci,2008, 83 (13/14) : 511-5. |
| [14] | Bolli R, Chugh AR, D'amario D, et al. Cardiac stem cell infusion in patients with ischemic cardiomyopathy(SCIPIO)[J]. Lancet,2011, 378 (9806) : 1847-57. DOI: 10.1016/S0140-6736(11)61590-0. |
| [15] | Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiospherederived cells for heart regeneration after myocardial infarction (CADUCEUS):a prospective, randomised phase 1 trial[J]. Lancet,2012, 379 (9819) : 895-904. DOI: 10.1016/S0140-6736(12)60195-0. |
| [16] | Edelberg JM, Lee SH, Kaur M, et al. Platelet-derived growth factorAB limits the extent of myocardial infarction in a rat model:feasibility of restoring impaired angiogenic capacity in the aging heart[J]. Circulation,2002, 105 (5) : 608-13. DOI: 10.1161/hc0502.103672. |
| [17] | Davis DR, Ruckdeschel Smith R, Marbán E. Human cardiospheres are a source of stem cells with cardiomyogenic potential[J]. Stem Cells,2010, 28 (5) : 903-4. |
| [18] | Aghila Rani KG, Kartha CC. Effects of epidermal growth factor on proliferation and migration of cardiosphere-derived cells expanded from adult human heart[J]. Growth Factors,2010, 28 (3) : 157-65. DOI: 10.3109/08977190903512628. |
| [19] | Gaetani R, Ledda M, Barile L, et al. Differentiation of human adult cardiac stem cells exposed to extremely low-frequency electromagnetic fields[J]. Cardiovasc Res,2009, 82 (3) : 411-20. DOI: 10.1093/cvr/cvp067. |
| [20] | Mishra R, Vijayan K, Colletti EJ, et al. Characterization and functionality of cardiac progenitor cells in congenital heart patients[J]. Circulation,2011, 123 (4) : 364-73. DOI: 10.1161/CIRCULATIONAHA.110.971622. |
| [21] | Takehara N, Tsutsumi Y, Tateishi K, et al. Controlled delivery of basic fibroblast growth factor promotes human cardiospherederived cell engraftment to enhance cardiac repair for chronic myocardial infarction[J]. J Am Coll Cardiol,2008, 52 (23) : 1858-65. DOI: 10.1016/j.jacc.2008.06.052. |
| [22] | Tang YL, Zhu W, Cheng M, et al. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression[J]. Circ Res,2009, 104 (10) : 1209-16. DOI: 10.1161/CIRCRESAHA.109.197723. |
| [23] | Zakharova L, Mastroeni D, Mutlu N, et al. Transplantation of cardiac progenitor cell sheet onto infarcted heart promotes cardiogenesis and improves function[J]. Cardiovasc Res,2010, 87 (1) : 40-9. DOI: 10.1093/cvr/cvq027. |
| [24] | Koninckx R, Daniels A, Windmolders S, et al. Mesenchymal stem cells or cardiacprogenitors for cardiac repair? A comparative study[J]. Life Sci,2011, 68 : 2141-56. |
 2016, Vol. 36
2016, Vol. 36
