2. 南方医科大学珠江医院肾内科,广东 广州 510280 ;
3. 中山市古镇人民医院肾内科,广东 中山 528421
2. Department of Nephrology, hujiang Hospital of Southern Medical University, Guangzhou 510280, China ;
3. Department of Nephrology, Zhongshan Guzhen People's Hospital, Zhongshan 528421, China
慢性肾脏病(CKD)已成为危害人类健康的严重公共卫生问题,肾间质纤维化是各种CKD进展至终末期肾衰竭的共同通路。研究表明[1-4],肾小管上皮细胞间充质转分化(EMT)在肾间质纤维化过程中起着至关重要的作用,但关于EMT的调控机制目前仍知之甚少。晚期氧化蛋白产物(advanced oxidation protein products,AOPPs)是新近发现的一类尿毒症毒素,主要成分为血清白蛋白酪氨酸残基氧化后形成的蛋白交联产物,由Witko Sarsat等[5]于1996年首次在慢性肾衰患者血浆中发现AOPPs水平在轻度的慢性肾功能不全患者循环中即已开始升高,且随着肾功能的恶化而进行性增高[6-7]。我们以往研究表明,AOPPs 可通过激活内质网应激(ERS)诱导肾小管上皮细胞发生EMT[8-9],且ERS与氧化应激有关,但其具体机制目前尚未完全明确。丝裂原活化蛋白激酶(MAPK)是细胞内的一类丝氨酸/苏氨酸蛋白激酶,与氧化应激密切相关[10-13]。p38丝裂原活化蛋白激酶(p38 MAPK)是MAPK其中一员,近年大量研究表明[14-19],p38 MAPK信号途径在介导多种刺激因素所致EMT过程中发挥着重要作用,但该信号途径是否参与了AOPPs诱导的肾小管上皮细胞EMT的过程目前尚不清楚。本文主要探讨p38 MAPK信号途径是否介导了AOPPs诱导肾小管上皮细胞发生EMT的过程,及探讨该途径与ERS的关系,旨在进一步阐明肾间质纤维化的机制。
1 材料和方法 1.1 细胞株与实验试剂HK-2细胞株为人肾小管上皮细胞的永生系,购自美国ATCC细胞库;胎牛血清(FBS)、DMEM/F12细胞培养液(美国Gibco 公司);无内毒素牛血清白蛋白(BSA,美国Sigma);p38 MAPK信号途径特异性阻断剂SB203580(美国Biomol);E-钙黏素(E-cadherin)和葡萄糖调节蛋白78(GRP78)一抗(美国Santa Cruz);波形蛋白(vimentin)、p38 MAPK及磷酸化p38 MAPK一抗(美国CST);ECL检测试剂盒(美国Millipore);Trizol试剂(美国Invitrogen);cDNA 第一链合成试剂盒和SYBR Premix Ex TaqTM 荧光定量PCR 试剂盒(日本TaKaRa);E-cadherin、vimentin、GRP78及内参照β-actin引物由生工生物工程上海(股份)有限公司合成。
1.2 方法 1.2.1 体外制备AOPP-BSA将不含游离氨基酸、碳水化合物、脂类成分的胎牛血清白蛋白(BSA)与200 mmol/L次氯酸按1∶140混合,避光室温反应30 min后,制备出不含糖基化终产物结构的AOPP-BSA。制备的AOPP-BSA在无菌PBS中透析过滤24 h以除去游离的HOCl,并过Detoxi-Gel柱去除内毒素。AOPPs含量通过测定酸性条件下340 nm的光吸收,以氯胺T为标准取得。通过上述方法制备的AOPP-BSA的AOPP含量为65.2±2.12 nmol/mg 蛋白,未经修饰的BSA中AOPP的含量为0.2±0.04 nmol/mg蛋白。制备的所有AOPP-BSA均经鲎实验法测定内毒素含量。内毒素含量低于0.25 EU/mL。
1.2.2 HK-2 细胞的培养将HK-2 细胞培养与含10%FBS的DMEM/F12 培养液中,胰酶消化后接种于6 孔板,换为无血清的DMEM/F12培养基中使细胞同步生长24 h。
1.2.3 实验分组为明确AOPPs对p38 MAPK信号通路的影响,将细胞分为3 组:(1)空白对照组(Ctrl 组);(2)BSA组:200 μg/mL BSA作用24 h;(3)AOPPs 组:200 μg/mLAOPPs作用24 h。
为明确p38 MAPK信号通路在AOPPs对HK-2细胞EMT及ERS过程中的作用,将细胞分为3组:(1)BSA组:200 μg/mL BSA作用24 h;(2)AOPPs组:200 μg/mLAOPPs作用24 h;(3)AOPPs+SB203580组:10 μmol/LSB203580预处理1 h后,予200 μg/mLAOPPs作用24 h。为明确ERS在AOPPs对p38 MAPK信号通路中的作用,将细胞分为3 组:(1)BSA组:200 μg/mL BSA作用24 h;(2)AOPPs组:200 μg/mL AOPPs作用24 h;(3)AOPPs + salubrinal(ERS 阻断剂)组:50 μmol/Lsalubrinal预处理1 h后,给予200 μg/mLAOPPs作用24 h。
1.2.4 实时荧光定量PCR按实验分组不同处理HK-2细胞后,用Trizol法提取各样本细胞总RNA,于紫外分光光度仪检测RNA含量及纯度。参照试剂盒说明书操作反转录合成cDNA。采用SYBR Premix Ex TaqTM荧光定量PCR试剂盒检测,管家基因β-actin作为内参,引物序列见表 1。反应条件为94 ℃预变性2 min,94 ℃变性15 s,60 ℃退火延伸30 s,扩增40个循环。上述实验重复3次。采用2-DDCt法进行统计分析。
| 表 1 基因引物序列及扩增产物大小 Table 1 Primer sequence for qRT-PCR |
细胞培养及干预方法同上。AOPPs作用24 h后用RIPA裂解细胞后提取细胞蛋白,采用BCA法测定蛋白浓度。行聚丙烯酰氨凝胶电泳后转膜至硝酸纤维膜。用5%BSA封闭2 h后加入一抗,4℃孵育过夜。吸弃一抗,TBST洗膜,15 min/次,共3次,然后用TBST 稀释二抗,室温杂交1 h。TBST 洗膜,15 min/次,共3 次,然后室温下ECL 显色。以β-actin 作为内参。Image J 软件分析确定杂交条带的相对吸光度值。
1.2.6 统计学方法各组实验均重复3次以上。计量数据以均数±标准差表示,多组间比较采用单因素方差分析。方差齐时,组间两两比较比较采用LSD法;方差不齐时,组间两两比较采用Dunnett's T3法。采用SPSS20.0统计分析软件进行数据分析。P<0.05视为差异有统计学意义。
2 结果 2.1 AOPPs对p38 MAPK信号通路的影响根据我们前期研究[8],我们将未经修饰的BSA(200 μg/mL)和AOPPs(200 μg/mL)分别刺激HK-2 细胞24 h。Western blotting的结果显示,与空白对照组相比,AOPPs可诱导p38 MAPK磷酸化(图 1,P<0.05),但未经修饰的BSA对p38 MAPK无明显影响(图 1)。
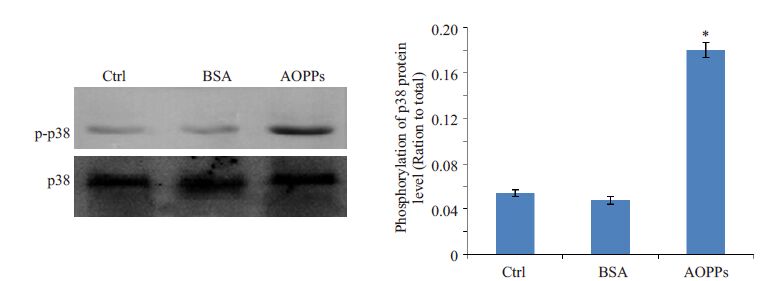
|
图 1 AOPPs对p38磷酸化水平的影响 Figure 1 Effect of AOPPs on phosphorylation of p38 MAPK in HK-2 cells. *P<0.05 vs control group. |
EMT的影响肾小管上皮细胞EMT是指肾小管上皮细胞在多种致病因素的作用下失去黏附性,并丢失标志性蛋白,如上皮型钙黏蛋白(E-cadherin),而表达间充质细胞的标志性蛋白,如波形纤维蛋白(vimentin),最终发生细胞表型转分化而成为肌成纤维细胞。本研究为了探讨p38MAPK信号途径是否介导了AOPPs诱导肾小管上皮细胞发生EMT的过程,予p38 MAPK信号通路的特异性阻断剂SB203580预处理HK-2细胞,然后与AOPPs共同孵育HK-2细胞,检测EMT的标志性蛋白E-cadherin和vimentin。实验结果显示:SB203580 可明显抑制AOPPs诱导的p38 MAPK磷酸化(图 2,P<0.05),并明显抑制AOPPs 下调的E-cadherin 的蛋白(图 3A)和mRNA(图 3B)水平表达(P<0.05),及明显抑制AOPPs上调的vimentin 的蛋白(图 3A)和mRNA(图 3C)水平表达(P<0.05),即SB203580可抑制AOPPs激活的p38MAPK 信号通路,及抑制AOPPs 诱导的HK-2 细胞EMT的发生。
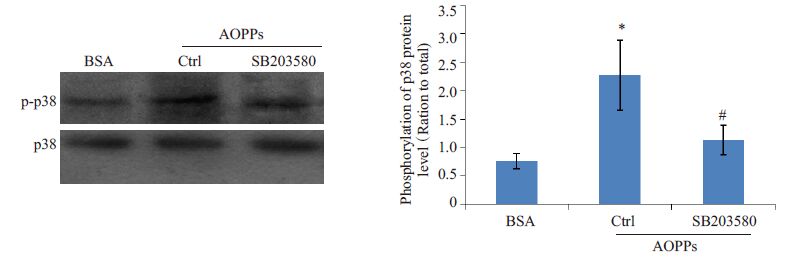
|
图 2 SB203580对AOPPs诱导p38磷酸化的影响 Figure 2 Effect of SB203580 on AOPP-induced phosphorylation of p38 MAPK in HK-2 cells. *P<0.05 vsBSA-treated cells,#P<0.05 vs AOPP-treated cells. |
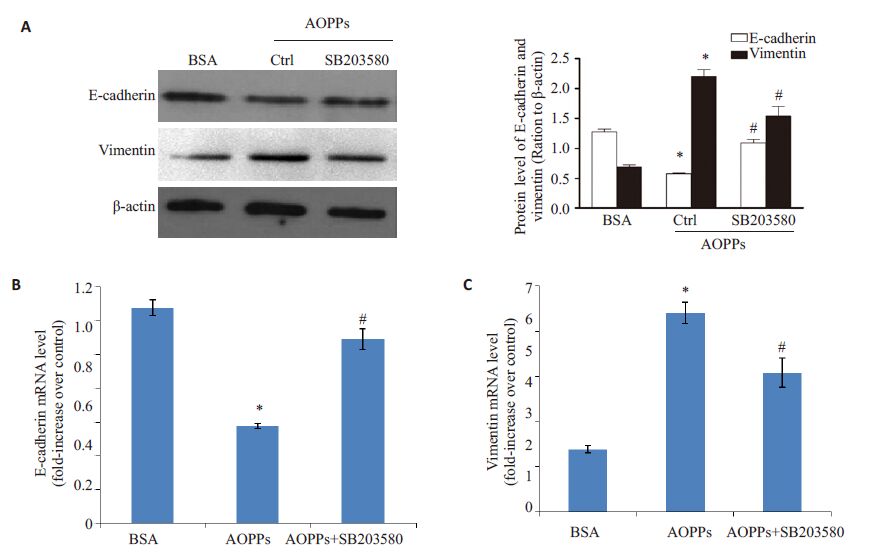
|
图 3 p38 MAPK信号通路在AOPPs所致HK-2细胞EMT过程中的影响 Figure 3 Effect of the p38 MAPK signaling pathway in on AOPPs-induced EMT in HK-2 cells. Western blotting (A) andqRT-PCR (B,C) results show the expression levels of E-cadherin and vimentin in HK-2 cells treated with BSA or AOPPs inthe presence or absence of SB203580. *P<0.05 vs BSA-treated cells,#P<0.05 vs AOPP-treated cells. |
由于各种原因引起的功能紊乱时出现错误折叠或未折叠蛋白在腔内聚集和Ca2+平衡紊乱的状态,称为ERS。我们前期研究已证明,AOPPs可通过激活ERS诱导肾小管上皮细胞发生EMT,本研究为了探讨此过程中p38 MAPK信号通路与ERS的关系,p38 MAPK信号通路的特异性阻断剂SB203580 预处理HK-2 细胞,然后予AOPPs刺激HK-2细胞,检测ERS激活的标志性蛋白GRP78的表达水平。Western blotting和荧光定量PCR的结果显示:SB203580可明显抑制AOPPs上调的GRP78的蛋白(图 4A)和mRNA(图 4B)水平表达(P<0.05),即SB203580可抑制AOPPs激活的ERS。
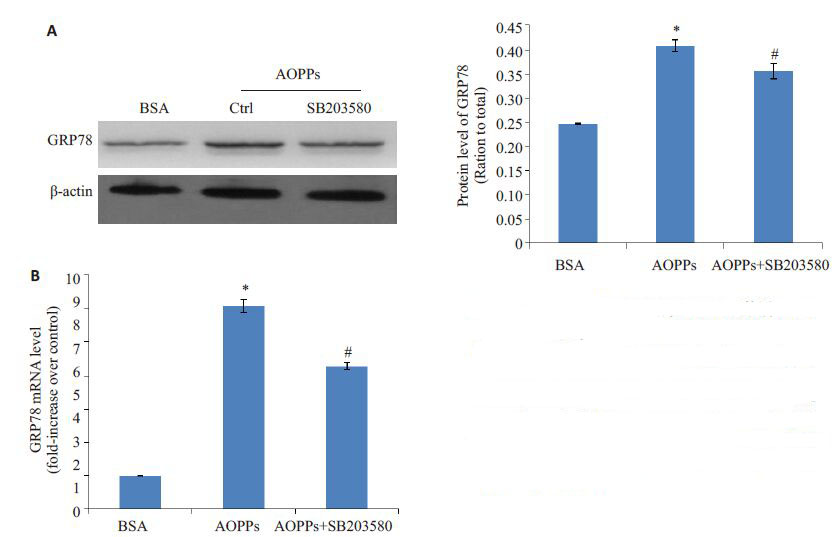
|
图 4 p38 MAPK信号通路在AOPPs 激活HK-2细胞ERS过程中的影响 Figure 4 Effect of the p38 MAPK signaling pathwayon AOPPs-induced ERS in HK-2 cells. Westernblotting (A) and qRT-PCR (B) results show theexpression of GRP78 in HK-2 cells treated withBSA or AOPPs in the presence or absence ofSB203580. *P<0.05 vs BSA-treated cells; #P<0.05 vs AOPP-treated cells. |
为了进一步探讨p38 MAPK信号通路与ERS的关系,我们将细胞分为3组:分别为:BSA组、AOPPs处理组、AOPPs+salubrinal(ERS的阻断剂)组。Western印迹法的结果显示:salubrinal可明显抑制AOPPs诱导的p38 MAPK 磷酸化(图 5),提示AOPPs 激活的p38MAPK受ERS的调节。
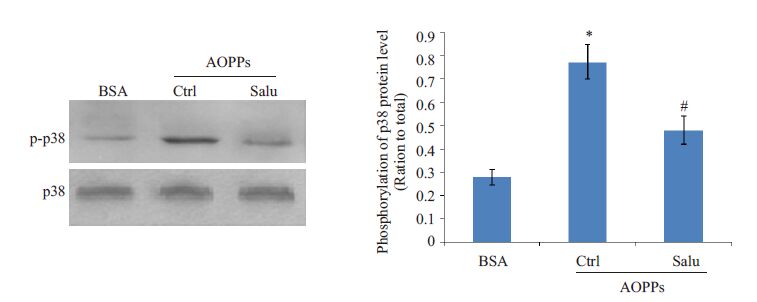
|
图 5 ERS对AOPPs激活p38 MAPK信号通路的影响 Figure 5 Effect of ERS on AOPPs-induced activation of p38 MAPK signaling pathway in HK-2 cells. *P<0.05 vs BSA-treated cells,#P<0.05 vs AOPP-treated cells. |
肾脏纤维化是各种CKD进展至终末期肾衰竭的共同通路,然而肾脏纤维化的机制十分复杂,目前仍未完全清楚。然而目前关于肾小管上皮细胞间充质转分化对于肾间质转分化的贡献程度仍有争议。Kriz等[20]研究者质疑肾小管上皮细胞转分化可致体内的肾脏纤维化;且近年的一项研究表明,在肾脏纤维化中,仅5%的成纤维细胞来源肾小管上皮细胞间充质转分化[21]。但近年来大量的研究表明,肾小管上皮细胞发生EMT在肾间质纤维化过程中发挥着重要作用[3-4, 22]。且有研究提示,肾小管上皮细胞发生EMT对肾脏纤维化作用程度不一可能是与实验条件有关,比如实验模型[23]。综上,肾小管上皮细胞EMT可促进肾间质纤维化,探讨肾小管上皮细胞EMT发生可望为延缓肾间质纤维化的进展提供新的干预靶点,具有十分重要的意义。
AOPPs作为一类促炎症、促氧化的大分子生物毒素,近年来,大量证据表明其与肾脏疾病的发生发展密切相关[6, 24-25]。我们前期研究已证明了AOPPs可通过激活ERS诱导肾小管上皮细胞发生EMT。本研究首次证明了p38 MAPK信号途径可能参与了AOPPs诱导肾小管上皮细胞发生EMT的过程。主要依据如下两点。首先,本实验结果显示,AOPPs 可诱导p38 MAPK磷酸化,但未经修饰的BSA对p38 MAPK无明显影响,表明AOPPs的刺激作用源于AOPP修饰而非BSA本身的作用,提示AOPPs可激活p38 MAPK信号途径;其次,本实验结果显示p38 MAPK信号途径的特异性阻断剂SB203580 可抑制AOPPs 诱导的EMT 的发生,提示p38 MAPK信号途径参与了AOPPs诱导HK-2细胞的EMT的发生。以往研究表明,p38 MAPK信号通路参与了多种刺激因素诱导的肾小管上皮细胞转分化的过程,如:高糖[18]、晚期糖基化终产物[17]等,但本研究首次提出了p38 MAPK信号通路介导了AOPPs诱导肾小管上皮细胞发生EMT的过程。
已知p38是MAPK的家族成员之一,而MAPK信号通路与氧化应激密切相关[12-13],且氧化应激与ERS密切相关[8, 26]。故本研究继续探讨在AOPPs诱导HK-2细胞发生EMT的过程中,p38 MAPK信号途径与ERS的关系。本研究结果提示,在AOPPs诱导HK-2细胞发生EMT 的过程中,p38 MAPK信号途径与ERS 密切相关。主要依据如下两点。首先,本实验结果提示,p38MAPK信号途径的特异性阻断剂SB203580 可明显抑制AOPPs激活的ERS,提示p38 MAPK信号途径参与了AOPPs激活肾小管上皮细胞ERS的过程;其次,ERS的阻断剂salubrinal 可部分抑制AOPPs 激活的p38MAPK信号途径,提示AOPPs激活的p38 MAPK信号途径可能受ERS的调节。综上,在AOPPs诱导HK-2细胞发生EMT的过程中,p38 MAPK信号途径与ERS密切相关,两者相互调节,但具体机制尚待进一步研究。
总之,本研究首次提出p38 MAPK信号途径参与了AOPPs诱导HK-2细胞的EMT的发生,且在此过程中,该信号途径与ERS的激活密切相关。本研究进一步阐明肾间质纤维化的机制。
| [1] |
Li Y, Sun Y, Liu F, et al. Norcantharidin inhibits renal interstitial fibrosis by blocking the tubular epithelial-mesenchymal transition[J].
PLoS One,2013, 8 (6) : e66356.
DOI: 10.1371/journal.pone.0066356. ( 0) 0)
|
| [2] |
Hay N, Sonenberg N. Upstream and downstream of mTOR[J].
Genes Dev,2004, 18 (16) : 1926-45.
DOI: 10.1101/gad.1212704. ( 0) 0)
|
| [3] |
Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis[J].
J Am Soc Nephrol,2010, 21 (2) : 212-22.
DOI: 10.1681/ASN.2008121226. ( 0) 0)
|
| [4] |
Carew RM, Wang B, Kantharidis P. The role of EMT in renal fibrosis[J].
Cell Tissue Res,2012, 347 (1) : 103-16.
DOI: 10.1007/s00441-011-1227-1. ( 0) 0)
|
| [5] |
Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia[J].
Kidney Int,1996, 49 (5) : 1304-13.
DOI: 10.1038/ki.1996.186. ( 0) 0)
|
| [6] |
Li HY, Hou FF, Zhang X, et al. Advanced oxidation protein products accelerate renal fibrosis in a remnant kidney model[J].
J Am Soc Nephrol,2007, 18 (2) : 528-38.
DOI: 10.1681/ASN.2006070781. ( 0) 0)
|
| [7] |
Liu B, Hou X, Zhou Q, et al. Detection of advanced oxidation protein products in patients with chronic kidney disease by a novel monoclonal antibody[J].
Free Radic Res,2011, 45 (6) : 662-71.
DOI: 10.3109/10715762.2011.564167. ( 0) 0)
|
| [8] |
Tang X, Rong G, Bu Y, et al. Advanced oxidation protein products induce hypertrophy and epithelial-to-mesenchymal transition in human proximal tubular cells through induction of endoplasmic reticulum stress[J].
Cell Physiol Biochem,2015, 35 (2) : 816-28.
DOI: 10.1159/000369740. ( 0) 0)
|
| [9] |
Tang X, Liang X, Li M, et al. ATF6 pathway of unfolded protein response mediates advanced oxidation protein product-induced hypertrophy and epithelial-to-mesenchymal transition in HK-2 cells[J].
Mol Cell Biochem,2015, 407 (1/2) : 197-207.
( 0) 0)
|
| [10] |
Mcclung JM, Judge AR, Powers SK, et al. p38 MAPK links oxidative stress to autophagy-related gene expression in cachectic muscle wasting[J].
Am J Physiol Cell Physiol,2010, 298 (3) : C542-9.
DOI: 10.1152/ajpcell.00192.2009. ( 0) 0)
|
| [11] |
Kita K, Sugita K, Sato C, et al. Extracellular release of annexin a2 is enhanced upon oxidative stress response via the p38 MAPK pathway after Low-Dose X-Ray irradiation[J].
Radiat Res,2016, 186 (1) : 79-91.
DOI: 10.1667/RR14277.1. ( 0) 0)
|
| [12] |
Ahad A, Ahsan H, Mujeeb M, et al. Gallic acid ameliorates renal functions by inhibiting the activation of p38 MAPK in experimentally induced type 2 diabetic rats and cultured rat proximal tubular epithelial cells[J].
Chem Biol Interact,2015, 240 : 292-303.
DOI: 10.1016/j.cbi.2015.08.026. ( 0) 0)
|
| [13] |
Qiu Y, Tao L, Lei C, et al. Downregulating p22phox ameliorates inflammatory response in Angiotensin II-induced oxidative stress by regulating MAPK and NF-kappaB pathways in ARPE-19 cells[J].
Sci Rep,2015, 5 : 14362.
DOI: 10.1038/srep14362. ( 0) 0)
|
| [14] |
Li Z, Liu X, Wang B, et al. Pirfenidone suppresses MAPK signaling pathway to reverse epithelial-mesenchymal transition and renal fibrosis[J].
Nephrology(Carlton),2016, 10 : 12831.
( 0) 0)
|
| [15] |
Wang L, Bai YY, Yang Y, et al. Diabetes mellitus stimulates pancreatic cancer growth and epithelial-mesenchymal transitionmediated metastasis via a p38 MAPK pathway[J].
Oncotarget,2015, 10 : 18632.
( 0) 0)
|
| [16] |
Rodrigues-Diez R, Carvajal-Gonzalez G, Sanchez-Lopez E, et al. Pharmacological modulation of epithelial mesenchymal transition caused by angiotensin II. Role of ROCK and MAPK pathways[J].
Pharm Res,2008, 25 (10) : 244-61.
( 0) 0)
|
| [17] |
Bai YH, Wang JP, Yang M, et al. SiRNA-HMGA2 weakened AGEsinduced epithelial-to-mesenchymal transition in tubular epithelial cells[J].
Biochem Biophys Res Commun,2015, 457 (4) : 730-5.
DOI: 10.1016/j.bbrc.2015.01.063. ( 0) 0)
|
| [18] |
Lv ZM, Wang Q, Wan Q, et al. The role of the p38 MAPK signaling pathway in high glucose-induced epithelial-mesenchymal transition of cultured human renal tubular epithelial cells[J].
PLoS One,2011, 6 (7) : e22806.
DOI: 10.1371/journal.pone.0022806. ( 0) 0)
|
| [19] |
Zeng R, Han M, Luo Y, et al. Role of Sema4C in TGF-β1-induced mitogen-activated protein kinase activation and epithelialmesenchymal transition in renal tubular epithelial cells[J].
Nephrol Dial Transplant,2011, 26 (4) : 1149-56.
DOI: 10.1093/ndt/gfq619. ( 0) 0)
|
| [20] |
Kriz W, Kaissling B. Le Hir M. Epithelial-mesenchymal transition (EMT)in kidney fibrosis: fact or fantasy[J] ? J Clin Invest, 2011, 121(2): 468-74.
( 0) 0)
|
| [21] |
Lebleu VS, Taduri G, O'connell J, et al. Origin and function of myofibroblasts in kidney fibrosis[J].
Nat Med,2013, 19 (8) : 1047-53.
DOI: 10.1038/nm.3218. ( 0) 0)
|
| [22] |
Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis[J].
J Clin Invest,2003, 112 (12) : 1776-84.
DOI: 10.1172/JCI200320530. ( 0) 0)
|
| [23] |
Inoue T, Umezawa A, Takenaka T, et al. The contribution of epithelial-mesenchymal transition to renal fibrosis differs among kidney disease models[J].
Kidney Int,2015, 87 (1) : 233-8.
DOI: 10.1038/ki.2014.235. ( 0) 0)
|
| [24] |
Zhou LL, Hou FF, Wang GB, et al. Accumulation of advanced oxidation protein products induces podocyte apoptosis and deletion through NADPH-dependent mechanisms[J].
Kidney Int,2009, 76 (11) : 1148-60.
DOI: 10.1038/ki.2009.322. ( 0) 0)
|
| [25] |
Iwao Y, Nakajou K, Nagai R, et al. CD36 is one of important receptors promoting renal tubular injury by advanced oxidation protein products[J].
Am J Physiol Renal Physiol,2008, 295 (6) : F1871-80.
DOI: 10.1152/ajprenal.00013.2008. ( 0) 0)
|
| [26] |
Santos CX, Tanaka LY, Wosniak J, et al. Mechanisms and implications of reactive Oxygen species Generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase[J].
Antioxid Redox Signal,2009, 11 (10) : 2409-27.
DOI: 10.1089/ars.2009.2625. ( 0) 0)
|
 2006, Vol. 36
2006, Vol. 36
