Psoralidin, 3, 9-dihydroxy-2-(3-methylbut-2-enyl)-[1]-benzofuro-[3, 2-c]-chromen-6-one, is a natural furanocoumarin isolated from Psoralea corylifolia L[1]with a wide spectrum of biological activities such as anticancer, antioxidant, antibacterial, antidepressant, and anti-inflammatory activities, and is also shown to regulate insulin signaling [2-6]. In vivo studies of psoralidin, however, are much limited by its poor water solubility and low absorbability[7]. Glycosylation provides an effective strategy to improve the water solubility, chemical stability, pharmacokinetic properties, and biological potency of many natural products [8-10]. In this study, we attempted to use in vitro enzymatic glycosylation for structural modification of psoralidin to enhance its water solubility and absorbability.
UDP-glycosyltransferase (YjiC) is a member of the GT1 family capable of transferring different types of activated sugars (NDP-sugar) to an acceptor. Recent studies have described the use of GTs (YjiC) from Bacillus licheniformis DSM-13 to synthesize novel glucosides such as mupirocin, apigenin, phloretin, resveratrol, geldanamycin analogs, isobavachalcone, and neolignan [11-18]. In this study, we report the in vitro glycosylation and examined the structure, water solubility, and stability of the novel psoralidin glucoside.
2 MATERIALS AND METHODS 2.1 Instruments and reagentsNuclear magnetic resonance (NMR) spectroscopic data were acquired on a Bruker AvanceⅡ600 NMR spectrometer (Bruker, Billerica, MA, USA). HR-ESI-MS spectra were recorded on an Agilent 6538 Accurate Q-TOF mass spectrometer (Agilent Technologies, USA). Semi-preparative reversed-phase high-performance liquid chromatography (HPLC) was carried out on 2535Q (Waters, USA). The YjiC enzyme expression vector (pET302-YjiC) was obtained from Prof. Jae Kyung Sohng of Sun Moon University. UDP-glucose and other reagents were purchased from Sigma-Aldrich (USA). HPLC-grade acetonitrile and methanol were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Psoralidin was isolated from P. corylifolia seeds with a purity exceeding 98%. The structure of psoralidin was confirmed by electrospray ionizationmass spectrometry (ESI-MS) and proton nuclear magnetic resonance spectroscopy (1H-NMR) of the reference substance[19].
2.2 Enzymatic glycosylation of novel psoralidin glucosideThe expression and purification of YjiC was described in detail previously [15-18]. For in vitro glycosylation of psoralidin, a total volume of 50 mL containing 100 mmol/L Tris-HCl (pH 9.6), 1 mmol/L MgCl2·6H2O, 1.5 mmol/L psoralidin, 3 mmol/L UDP-glucose, and 10 mL MeOH was mixed with 35μg/mL of YjiC. The reaction mixture was incubated at 30℃for 3 h and quenched twice with an equal volume of EtOAc. The mixture was then centrifuged at 10 000 r/min for 5 min to remove the denatured protein. The EtOAc layer was combined, dried and dissolved in methanol for further analysis and purification. Finally, purification of the product was carried out by semi-preparative HPLC with a SunFireTM C18 column (250 mm×10 mm, Waters, Milford, MA, USA) connected to a UV detector (254 nm) with 20% acetonitrile (CH3CN-H2O, 3.0 mL/min) to yield compound 1 (12 mg).
2.3 Water solubility determinationTo determine the water solubility of the compounds, an excess of psoralidin and compound 1 were dissolved in 400μL HPLC-grade water in eppendorf tubes at room temperature. An ultrasonic cleaner was used to maximize the solubility of each compound. After sonication at room temperature for 30 min and centrifugation at 12 000 r/min for 10 min to remove the insoluble material, the solutions were analyzed by HPLC to determine the concentration of the sample solution.
2.4 Stability determinationPsoralidin glucoside (1) was extracted and purified as described above. To determine the pH value and temperature sensitivity, psoralidin and compound 1 were dissolved in 200μL Tris-HCl buffer of various pH levels (6.0-9.6) at different temperatures (50-100℃) for 30 min. The compounds were first dissolved in 200μL Tris·HCl buffer at pH values of 6.0, 7.0, 8.0, 8.8, and 9.6 for 30 min at room temperature. Similarly, for temperature sensitivity determination, psoralidin and compound 1 were incubated at 50, 60, 70, 80, 90, and 100℃for 30 min at pH 8.8. Aliquots (20μL) were used for HPLC analysis to determine the sample solution concentration. The stability of the compounds was calculated as a percentage of the total peak area.
2.5 MTT colorimetric assayHuman hepatocellular carcinoma (SMMC7721), breast cancer (MCF-7), and colon adenocarcinoma (SW480) cell lines were grown in DMEM media containing 10% fetal bovine serum (Hyclone, Logan, UT, USA) and 1% penicillin/streptomycin (Gibco, Grand Island, NY, USA). All the cell lines were maintained at 37℃in a humidified 5% CO2 incubator. To assess the anti-proliferative activity of the compounds, the cells were seeded in 96-well plates at a density of 6000 cells/ well for 1 day, and pre-incubated with the compounds at varying concentrations for 48 h. The anti-proliferative activity of the compounds was evaluated using standard MTT assay procedures[15, 18].
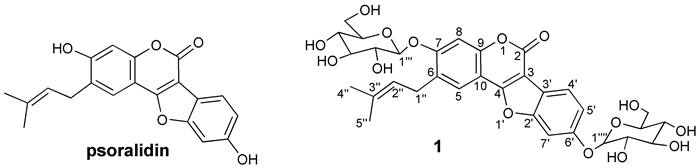
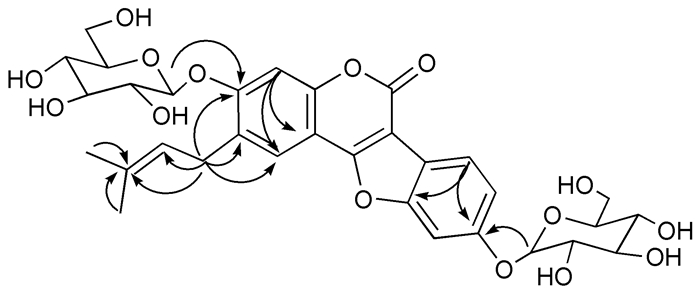
3 RESULTS 3.1 Identification of psoralidin glucosideIn vitro enzymatic glycosylation was performed and the reaction products were purified by semi-prep HPLC to yield compound 1 (12 mg). Compound 1 was obtained as a white powder, and its molecular formula C32H36O15 was established by HR-ESI-MS at m/z 661.2137[M + H]+. The 1H-and 13C-NMR spectra of compound 1 were similar to those of the substrate, psoralidin [19]. In contrast, the NMR data of compound 1 showed two anomeric H-atoms δH 5.04 (1H, d, J=7.4 Hz, H-1'''), 5.00 (1H, d, J=7.3 Hz, H-1'''') and corresponding C-atoms δC 100.5 (C-1'''), 100.7 (C-1''''), indicating the presence of a disaccharide moiety (Tab. 1). The two glucopyranosyl moieties were attached to C-7 and C-6' based on the HMBC correlations from proton at δH 5.04 (H-1''') to δC 158.2 (C-7) and δH 5.00 (H-1'''') to δC 156.7 (C-6'). The HMBC profile also revealed a correlation between the δH 3.42 (2H, m, H-1'') to δC 120.8 (C-5), 128.3 (C-6), 158.2 (C-7), showing that the prenyl group was attached to C-6 (Fig. 1). In addition, both sugars showed aβ-conformation, based on the coupling constant of the anomeric proton at δH 5.04 (J=7.4 Hz) and 5.00 (J=7.3 Hz). Therefore, the structure of compound 1 was identified as psoralidin-6', 7-di-O-β-D-glucopyranoside (Fig. 2).
| Table 1 1H-and 13C-NMR (600/150 MHz) data of compound 1 in DMSO-d6 |
|
Figure 1 Key HMBC correlations of compound 1. |
|
Figure 2 Chemical structures of psoralidin and compound 1. |
The water solubility of the new glucoside (1) was evaluated by comparison with that of the substrate. The solubility of compound 1 in water was found to be 527.6±3.42μmol/L, approximately 32.6-fold higher than that of its substrate, psoralidin (16.23±2.31μmol/L). As expected, the enzymatic biosynthesis of a novel glucoside of psoralidin greatly enhanced its water solubility.
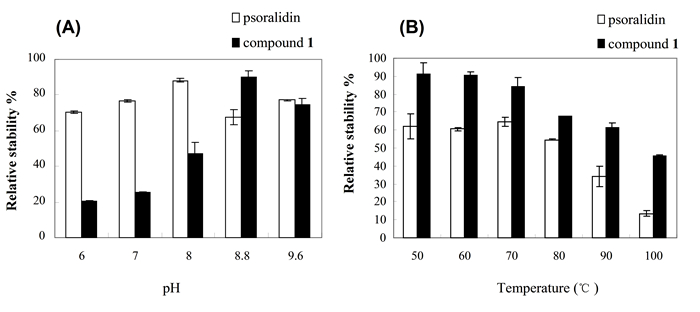
3.3 Determination of pH and temperature stabilityThe pH stability and temperature stability of the compounds were determined by incubating psoralidin and its glucoside (1) dissolved in 200μL Tris-HCl buffer at varying pH levels for 30 min at room temperature, and by incubating the compound solutions at different temperatures for 30 min at the most stable pH (8.8). HPLC analysis showed that the novel psoralidin glucoside (1) exhibited the highest stability at pH 8.8 with a good temperature stability at 50-70℃(Fig. 3). These results suggested that psoralidin glucoside is relatively stable at pH 8.8 and at high temperatures.
|
Figure 3 Determination of pH (A) and temperature (B) sensitivities of psoralidin and its glucoside (1). |
We next investigated the anti-proliferative activity of psoralidin and compound 1 against 3 cancer cell lines using MTT colorimetric assay. The results showed that 48 h after addition of the compounds in the cell culture, only psoralidin exhibited a moderate cytotoxicity against the 3 cancer cell lines with IC50 values ranging from 17.46 to 22.62μmol/L (Tab. 2).
| Table 2 Anti-proliferative activity (IC50) of the compounds against 3 cancer cell lines (µmol/L, Mean±SD, n=3) |
While natural products provide a rich source of therapeutically useful compounds, the pharmaceutical industry appears to show reduced interest in the development of natural products[20, 21]. Moreover, because most natural products display poor physicochemical and pharmacokinetic properties, optimization efforts would preclude semi-synthetic, genetic engineering, biotransformation or efficient route for structure-activity relationship studies.
Glycosylation of secondary metabolites is one of the most common modifications in plants and other produces to confer such physical changes as in water solubility and stability. However, some reactions including glycosylation, which is generally considered to enhance the water solubility, stability, and bioavailability of the substrate, are not easily accessible using chemical methods [22]. Chemical synthetic approaches for the production of glycosylated compounds involve tedious, time-consuming multi-step chemical reactions. Enzymatic glycosylation method using YjiC offers another option to produce psoralidin glucosides. So far as we know, this is the first report of in vitro enzymatic glycosylation of psoralidin using YjiC.
Glycosylation of natural products has emerged as a viable strategy for producing bioactive compounds with an improved activity [23]. Such sugar attachments also substantially influence pharmacological and pharmacokinetic properties, including tissue specificity, water solubility, distribution, and metabolic stability [22-24]. The novel psoralidin glucoside we obtained exhibited the highest stability at a pH 8.8 and at 50-70℃. Therefore, an extraction pH of 8.8 and a temperature around 60℃can be optimal conditions for biosynthesis of psoralidin glucoside.
To evaluate the pharmaceutical potency, we investigated the anti-proliferative activities of psoralidin and compound 1 against 3 cancer cell lines. Psoralidin is a member of the furanocoumarin subclass of coumarin, and consists of phenolic hydroxyl groups at the C-7 and C-6' positions. C7-OH and C6'-OH form important networks at the active position for biological activities, including antioxidant, antibacterial and anticancer activities [25]. Thus, the in vitro antiproliferative activity of compound 1 decreased considerably (IC50>200μmol/L), possibly as a consequence of the bulkiness of glycosylation at the C-7 hydroxyl group and the C-6' hydroxyl group[15, 26].
5 CONCLUSIONWe report the in vitro glycosylation of psoralidin by enzymatic biosynthesis. Our data suggest that YjiC is a glycosyltransferase that confers modifications to psoralidin and that glycosylation can improve the water solubility and stability of compounds. Further studies are needed to clarify whether compound 1 has antitumor activity in vivo.
| [1] |
Liu XY, Nam JW, Song YS, et al. Psoralidin, a coumestan analogue, as a novel potent estrogen receptor signaling molecule isolated from Psoralea corylifolia[J].
Bioorg Med Chem Lett,2014, 24 (5) : 1403-6.
DOI: 10.1016/j.bmcl.2014.01.029. ( 0) 0)
|
| [2] |
Yang HJ, Youn HS, Seong KM, et al. Psoralidin, a dual inhibitor of COX-2 and 5-LOX, regulates ionizing radiation (IR)-induced pulmonary inflammation[J].
Biochem Pharmacol,2011, 82 (5) : 524-34.
DOI: 10.1016/j.bcp.2011.05.027. ( 0) 0)
|
| [3] |
Jan S, Parween T, Siddiqi TO, et al. Anti-oxidant modulation in response to gamma radiation induced oxidative stress in developing seedlings of Psoralea corylifolia L[J].
J Environ Radioactiv,2012, 113 (11) : 142-9.
( 0) 0)
|
| [4] |
Wang TX, Yin ZH, Zhang W, et al. Chemical constituents from Psoralea corylifolia and their antioxidantα-glucosidase inhibitory and antimicrobial activities[J].
Zhongguo Zhong Yao Za Zhi,2013, 38 (14) : 2328-33.
( 0) 0)
|
| [5] |
Jin ZL, Yan W, Jin H, et al. Differential effect of psoralidin in enhancing apoptosis of colon cancer cells via nuclear factor-kappaB and B-cell lymphoma-2/B-cell lymphoma-2-associated X protein signaling pathways[J].
Oncol Lett,2016, 11 (1) : 267-72.
( 0) 0)
|
| [6] |
Hao WH, Zhang XN, Zhao WW, et al. Psoralidin induces autophagy through ROS generation which inhibits the proliferation of human lung cancer A549 cells[J].
PeerJ,2014, 2 : e555.
DOI: 10.7717/peerj.555. ( 0) 0)
|
| [7] |
Pahari P, Rohr J. Total synthesis of psoralidin, an anticancer natural product[J].
J Org Chem,2009, 74 (7) : 2750-4.
DOI: 10.1021/jo8025884. ( 0) 0)
|
| [8] |
Ghimire GP, Koirala N, Pandey RP, et al. Modification of emodin and aloe-emodin by glycosylation in engineered Escherihia coli[J].
World J Microb Biot,2015, 31 (4) : 611-9.
DOI: 10.1007/s11274-015-1815-4. ( 0) 0)
|
| [9] |
Nguyen HT, Jae KS. Recent biotechnological progress in enzymatic synthesis of glycosides[J].
J Ind Microbiol Biot,2013, 40 (12) : 1329-56.
DOI: 10.1007/s10295-013-1332-0. ( 0) 0)
|
| [10] |
Li Z, Wang J, Zhou Y, et al. Lead compound optimization strategy (3): structure modification strategies for improving water solubility[J].
Acta Pharm Sin,2014, 49 (9) : 1238-47.
( 0) 0)
|
| [11] |
Parajuli P, Pandey RP, Pokhrel AR, et al. Enzymatic glycosylation of the topical antibiotic mupirocin[J].
Glycoconjugate J,2014, 31 (8) : 563-72.
DOI: 10.1007/s10719-014-9538-6. ( 0) 0)
|
| [12] |
Gurung RB, Kim EH, Oh T, et al. Enzymatic synthesis of apigenin glucosides by glucosyltransferase (YjiC) from Bacillus licheniformis DSM 13[J].
Mol Cells,2013, 36 (4) : 355-61.
DOI: 10.1007/s10059-013-0164-0. ( 0) 0)
|
| [13] |
Pandey RP, Li TF, Kim EH, et al. Enzymatic synthesis of novel phloretin glucosides[J].
Appl Environ Microb,2013, 79 (11) : 3516-21.
DOI: 10.1128/AEM.00409-13. ( 0) 0)
|
| [14] |
Pandey RP, Parajuli P, Shin JY, et al. Enzymatic biosynthesis of novel resveratrol glucoside and glycoside derivatives[J].
Appl Environ Microb,2014, 80 (23) : 7235-43.
DOI: 10.1128/AEM.02076-14. ( 0) 0)
|
| [15] |
Wu CZ, Jang JH, Woo M, et al. Enzymatic glycosylation of nonbenzoquinone geldanamycin analogs via Bacillus UDPglycosyltransferase[J].
Appl Environ Microb,2012, 78 (21) : 7680-6.
DOI: 10.1128/AEM.02004-12. ( 0) 0)
|
| [16] |
Huo Q, Li HM, Lee JK, et al. Biosynthesis of novel glucosides geldanamycin analogs by enzymatic synthesis[J].
J Microbiol Biotechnol,2015, 26 (1) : 56-60.
( 0) 0)
|
| [17] |
Li HM, Lee JK, Nie LJ, et al. Enzymatic synthesis of novel isobavachalcone glucosides via a UDP-glycosyltransferase[J].
Arch Pharm Res,2015, 38 (12) : 2208-15.
DOI: 10.1007/s12272-015-0658-8. ( 0) 0)
|
| [18] |
Li HM, Li J, Jin W, et al. Transglycosylation of neolignans by enzymatic synthesis and evaluation of their antitumor activity[J].
J South Med Univ,2015, 35 (11) : 1570-4.
( 0) 0)
|
| [19] |
Xiao GD, Li GW, Chen L, et al. Isolation of antioxidants from Psoralea corylifolia fruits using high-speed counter-current chromatogramphy guided by thin layer chromatography-antioxidant autographic assay[J].
Chromatogr A,2010, 1217 (34) : 5470-6.
DOI: 10.1016/j.chroma.2010.06.041. ( 0) 0)
|
| [20] |
Quinn RJ, Carroll AR, Pham NB, et al. Developing a drug-like natural product library[J].
J Nat Prod,2008, 71 (3) : 464-8.
DOI: 10.1021/np070526y. ( 0) 0)
|
| [21] |
Galm U, Shen B. Natural product drug discovery: the times have never been better[J].
Chem Biol,2007, 14 (10) : 1098-104.
DOI: 10.1016/j.chembiol.2007.10.004. ( 0) 0)
|
| [22] |
Gantt RW, Peltier-Pain P, Thorson JS. Enzymatic methods for glycol (diversification/randomization) of drugs and small molecules[J].
Nat Prod Rep,2011, 28 (11) : 1811-53.
DOI: 10.1039/c1np00045d. ( 0) 0)
|
| [23] |
Singh S, Phillips GN, Thorson JS. The structural biology of enzymes involved in natural product glycosylation[J].
Nat Prod Rep,2012, 29 (10) : 1201-37.
DOI: 10.1039/c2np20039b. ( 0) 0)
|
| [24] |
Blanchard S, Thorson JS. Enzymatic tools for engineering natural product glycosylation[J].
Curr Opin Chem Biol,2006, 10 (3) : 263-71.
DOI: 10.1016/j.cbpa.2006.04.001. ( 0) 0)
|
| [25] |
Bubols GB, Vianna DR, Medina-Remón A, et al. The antioxidant activity of coumarins and flavonoids[J].
Mini-Rev Med Chem,2013, 13 (3) : 318-34.
( 0) 0)
|
| [26] |
Cheng, H, Cao XH, Xian M, et al. Synthesis and enzyme specific activation of carbohydrate geldanamycin conjugates with potent anticancer activity[J].
J Med Chem,2005, 48 (2) : 645-52.
DOI: 10.1021/jm049693a. ( 0) 0)
|
 2016, Vol. 36
2016, Vol. 36
