2解放军第323医院妇产科,陕西西安710032
2Department of Obstetrics and Gynecology, 323 Hospital of PLA, Xi'an 710032, China
胚胎的着床造成内膜微环境的炎症状态,蜕膜免疫细胞大量募集,巨噬细胞约占其中20%[1]。对反复着床失败的患者人工诱导无菌性炎症反应,巨噬细胞的明显聚集能促进胚胎着床,增加妊娠率,但其具体机制尚不明确[2, 3]。着床窗小鼠子宫内膜的微血管环境改变是子宫内膜容受性建立的关键[4]。在与胚胎着床相似的肿瘤侵袭过程中,肿瘤相关性巨噬细胞在维持肿瘤局部毛细血管网的平衡中发挥重要作用,其血管内皮生长因子A(VEGFA)是其最主要的促血管生长因子[5, 6, 7, 8]。由于胚胎着床与肿瘤侵袭过程类似,我们猜测子宫巨噬细胞也通过VEGFA参与了蜕膜局部血管生成微环境的构建,从而影响胚胎着床。因此,我们采用宫腔内注射消除巨噬细胞的药物氯膦酸二钠的脂质体(clodronateliposomes,CL)的方法,在小鼠的着床期抑制子宫内膜局部的巨噬细胞(macrophages,Mφ),观察其对子宫内膜VEGFA表达及胚胎着床的影响,以探讨其参与子宫内膜容受性调控及胚胎着床的主要机制。
1 材料和方法 1.1 试剂CL由荷兰Van Rooijen教授构建,浓度为5 mg/mL。APC 标记抗小鼠F4/80 抗体(ebioscience),PE 标记的Anti-mouseCD11b(ebioscience),SABC 试剂盒,DAB显色剂,内源性过氧化物酶抑制剂,PBS,枸橼酸盐缓冲液,4%多聚甲醛,胎牛血清,HEPES溶液,胶原蛋白酶I型,脱氧核糖核酸酶1(DNase1)。
1.2 实验动物性发育成熟未育的昆明种小鼠,由南方医科大学动物实验中心提供,适应性被饲养7 d后,进入实验,自然光照,自由饮水取食。雌鼠:6~8周龄,体质量28±2 g;雄鼠:2~3月龄性成熟。每日5∶00 pm将雌雄小鼠按照2∶1的比例合笼,次日8∶00 am检查小鼠阴道,发现阴栓定为D0.5。
1.3 主要仪器微量注射器、石蜡切片机、石蜡包埋机、生物组织烤片机、光学显微镜、镊子、剪刀、解剖盘等解剖用具,体式显微镜,BD ISRFortessa 流式细胞仪。
1.2.1 宫腔内脂质体注射取30只见栓小鼠,随机分为3组,每组10只。于第3.5孕鼠腹腔注射1%戊巴比妥充分麻醉后固定,使用微量注射器从近卵巢侧子宫角部向宫腔内缓慢注入15 μL药物。实验组(E)向左侧宫腔内注射CL,右侧注射包裹有磷酸缓冲盐的脂质体(PBSliposomes,PL);对照组(C)向双侧宫腔内注射PL;空白组(B)向双侧宫腔内注射等体积灭菌PBS溶液作为空白对照。术毕缝合消毒皮肤。
1.2.2 统计胚胎着床数于第5.5天处死前采取尾静脉注射台盼兰以示着床位点。5 min后断颈处死小鼠,取出子宫,观察各组小鼠妊娠情况和胚胎着床数。
1.2.3 免疫组织化学法(SABC法)各组注射后48 h,即D5.5,取子宫及卵巢,多聚甲醛固定后制作石蜡标本。据台盼兰示踪的着床位点处,连续切片至胚胎着床位点,厚度4 μm,各样本胚胎着床位点、着床位点之间的非着床位点1张切片作为代表。之后进行SABC法染色,每张切片随机取10个视野,在10×40倍镜下计数F4/80阳性Mφ。VEGFA阳性细胞胞膜可见棕黄色或棕褐色颗粒。VEGFA的表达情况采用半定量分析方法进行H评分:对每一个切片随机选取5个高倍镜视野(×400)计数200个细胞,在光镜下判断其胞浆的DAB染色强度,分别以0、1、2、3代表阴性、弱染色、中等染色及强染色等4个等级,H分值=[(弱染色强度细胞百分数×1)+(中度染色程度细胞百分数×2)+(强染色强度细胞百分数×3)]×100,H评分介于0~300之间。
1.2.4 流式细胞计数采用机械酶联合消化法,将新鲜子宫组织剪成l~2 mm3的小块,置于5 mL消化液(RPMI+10% FBS+0.015 mol/L HEPES+2000 U/mL胶原蛋白酶Ⅰ型+ 25 μg/mL DNase1)中,在37 ℃条件下消化2 h,制备子宫组织的单细胞悬液。加入F4/80-APC、CD11b-PE及0.5%BSA,避光4 ℃孵育20 min。用BD ISRFortessa 流式细胞仪行流式细胞学检测。
1.2.5 统计学分析应用SPSS16.0软件统计数据,计数结果用均数±标准差表示,Mφ数、胚胎着床数各组间比较采用完全随机设计资料的方差分析,方差齐选用LSD方法,方差不齐则用Duimett T3法。设双侧检验,P<0.05为差异有统计学意义。各组着床位点和非着床位点内膜的VEGFA的H值半定量资料均用中位数(最小值,最大值)表示,组间比较采用Kruskall-Wallis检验。以P<0.05认为差异有统计学意义。
2 结果 2.1 小鼠宫腔内注射CL后抑制小鼠子宫内膜局部的Mφ 2.1.1 胚胎着床位点和非着床位点子宫内膜Mφ、卵巢巨噬细胞的分布变化SABC法DAB显色后可见F4/80-Mφ呈棕黄色或棕褐色,阳性颗粒定位于Mφ膜质上,与背景色对比明显。呈圆形、椭圆形或不规则形状的Mφ,主要分布于腺体周围,少量散在分布于胚胎周围的蜕膜化区域(图1)。着床位点上,E组左侧的子宫内膜Mφ数(22.50±8.15)较PL侧(72.20±14.11)显著降低,较对照组左侧和空白组左侧也显著降低(P<0.05),3组的右侧则无明显差异。非着床位点Mφ数分布变化类似。除外E组左侧,其他子宫着床位点的巨噬细胞均高于非着床位点(P<0.05,表1)。
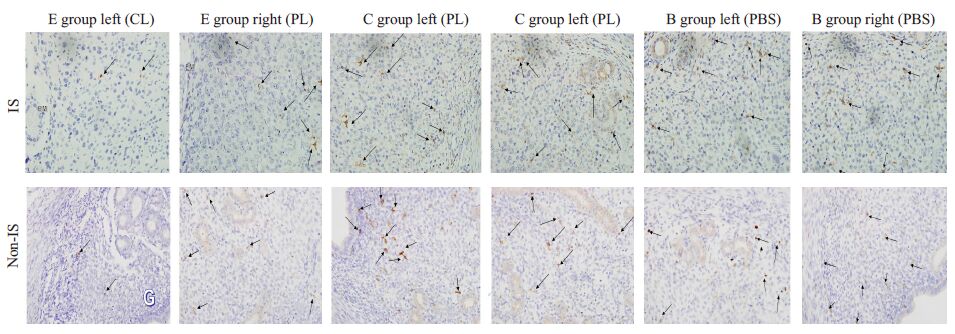
|
图1 宫腔内注射后小鼠子宫内膜着床点和非着床点F4/80+Mφ的分布变化 Fig.1 Changes of macrophages in mouse endometrium after intrauterine injection (SABC staining,original magnification: ×400). CL:Clodronate liposomes; PL: PBS liposomes; IS: implantation sites; Non-IS: Non-implantation sites. |
| 表1 宫腔内注射后子宫内膜及卵巢上F4/80+Mφ的数量变化 Table 1 Comparison of the number of F4/80+ macrophages in mouse endometrium and ovaries after intrauterine injection (Mean±SD) |
宫腔内注射CL后48 h,获取各组小鼠子宫组织并消化为单细胞悬液后进行F4/80和CD11b双阳性染色胞相对计数检测,显示实验组F4/80+CD11b+Mφ显著下降,抑制率为74%,与免疫组化中观察的结果相符,典型流式结果如图2所示。
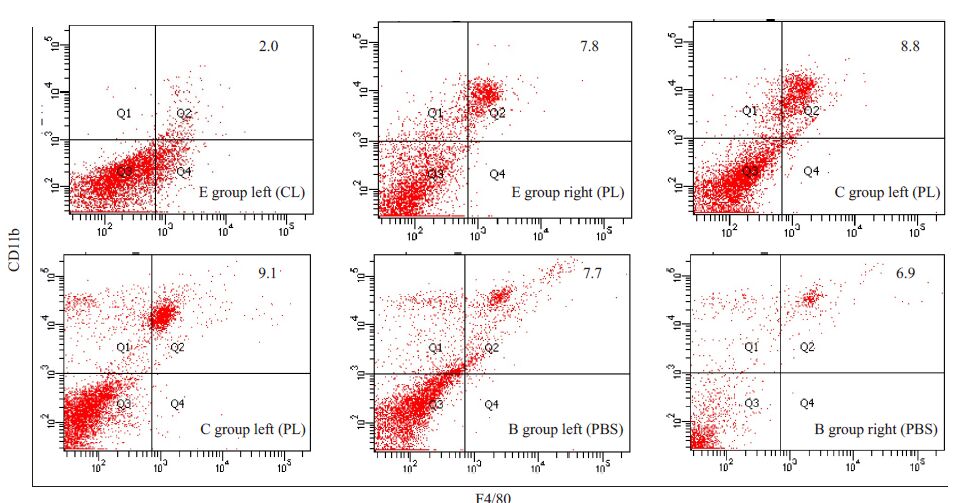
|
图2 宫腔内注射后流式细胞学方法检测子宫局部F4/80+CD11b+Mφ相对计数 Fig.2 FACS with anti-CD11b and anti-F4/80 antibodies showing macrophages after intrauterine injection. FACS plots are representative of10 mice per group. |
3组小鼠的各侧卵巢均可见多量散在Mφ,主要分布于基质和血管周围,E组卵巢的Mφ数量与C组、B组相比无明显差异(图3,表2)。
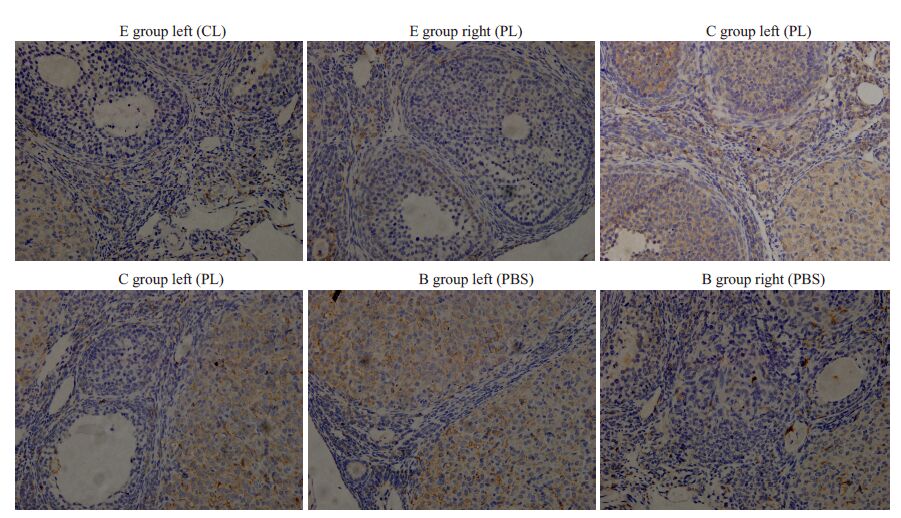
|
图3 宫腔内注射后小鼠卵巢F4/80阳性Mφ的分布变化 Fig.3 Changes of macrophage distribution in mouse ovaries after injections (SABC staining, original magnification: ×400). |
| 表2 宫腔内注射后小鼠子宫VEGFA的表达变化 Table 2 Comparison of the expression of VEGFA in mouse uterus after intrauterine injection (n=10) |
D5.5台盼兰染色通过显示血管通透性增加的着床位点计数,显示E组左侧平均胚胎着床数为2.20±1.81个,显著低于E组右侧、C组及B组左侧,平均着床位点分别:5.10±1.91 个,5.00±2.21,5.80±2.25(P<0.05);3组右侧无明显差异(图4)。
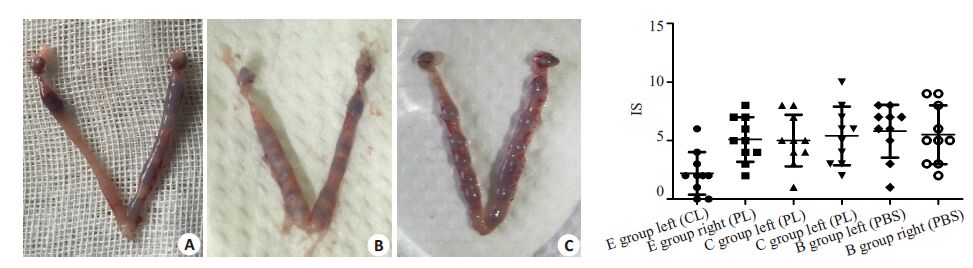
|
图4 宫腔内注射后的胚胎着床数 Fig.4 Number of implantation sites after intrauterine injection. A: E group; B: Cgroup; C: B group. |
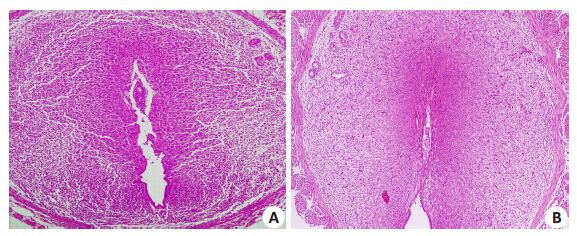
由孕鼠着床位点子宫横切面观察其组织学结构,镜下可见:3组子宫组织结构完整,腺体结构清晰,上皮细胞明显水肿,基质疏松,无炎性细胞浸润。实验组子宫内膜增厚、疏松,可见间质细胞蜕膜化区域,但范围较对照组与空白组缩小,仅有的胚胎着床位点,宫腔未闭合,血管增生较少。空白组、对照组小鼠子宫内膜,间质细胞明显蜕膜化,宫腔闭合,着床位点可见胚胎结构(图4)。
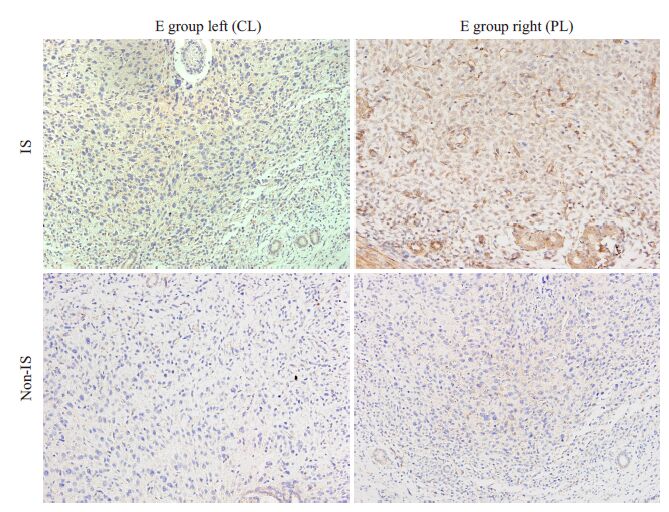
2.3 子宫内膜VEGFA的表达情况VEGFA阳性颗粒呈现黄色或棕黄色颗粒,定位于内膜子宫腔上皮和腺上皮及血管层里。
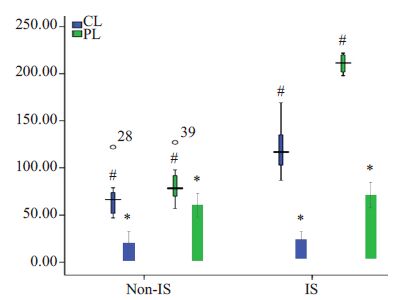
在着床位点,随着E组左侧注射CL后子宫Mφ受到抑制,子宫内膜的VEGFA阳性颗粒明显少于注射PL的E组右侧(P<0.05,图6,图7)。非着床点E组注射CL侧的子宫VEGFA的表达虽有所降低,但差异无统计学意义(P=0.069),E组两侧的VEGFA表达在着床位点的均高于非着床位点,差异有统计学意义(P<0.05,表2)。
|
图5 宫腔内注射后小鼠子宫HE染色 Fig.5 Histological changes of the uterus after intrauterine injection (HE staining,original magnification: ×100). A: CL; B: PL.缺失显著影响胚胎着床。 |
|
图6 宫腔内注射后小鼠子宫VEGFA的表达变化 Fig.6 Change of VEGFA expression in mouse uterus after intrauterine injection (SABC staining, original magnification: ×400). |
|
图7 宫腔内注射后小鼠子宫非着床位点和着床位点Mφ、VEGFA的表达变化 Fig.7 Change of VEGFA expression and macrophages at the implantation sites (IS) and non-implantation sites (nonIS) in mouse uterus after intrauterine injection. *: Mφ; #: VEGFA. |
围着床期子宫内膜Mφ募集数量具有明显的时间-空间依赖性,并分泌促炎抑炎因子。我们在前期研究中发现,着床期子宫Mφ数目与内膜蜕膜化过程中的血管生成呈相关性增加[9, 10]。为进一步明确巨噬细胞参与胚胎着床的机制,Care曾尝试全身敲除巨噬细胞来研究其在胚胎着床中的作用,但发现卵巢巨噬细胞同时被敲除,影响排卵而且干预了妊娠维持最为重要的孕激素,不能说明Mφ本身在着床期子宫内膜局部发挥的特定作用[11]。CL可特异性被Mφ吞噬,Mφ的磷脂酶破坏脂质体后氯膦酸二钠被释放到胞质内,达到特定浓度即可引起Mφ的凋亡[12]。CL能有效剔除眼部[13]、脑部[14]、卵巢[15]的Mφ,但应用于子宫局部未见报道。我们利用直视下宫腔内注射CL,消除单侧子宫的巨噬细胞,在国内外首次建立了小鼠子宫局部的Mφ抑制动物模型:流式细胞检测其巨噬细胞数量显著下降,抑制率达74%,免疫组化显示着床点和非着床点的巨噬细胞均有明显下降,而卵巢巨噬细胞未受影响。该方法实现同一小鼠左右侧子宫的自身对照,更好的避免了个体间差异;对照组、空白组的子宫巨噬细胞无明显降低,排除了脂质体本身及手术操作对实验的影响;局部作用与子宫,避免了对卵巢巨噬细胞及孕激素的影响,为理想的子宫局部巨噬细胞抑制模型。
通过该模型我们发现,在着床期抑制巨噬细胞后,发现不仅胚胎着床数减少了42.60%,且注射侧的子宫仅有的着床位点分布不均匀,这与Pollard报道的巨噬细胞集落刺激因子(CSF-1)基因缺失的小鼠巨噬细胞数量下降导致妊娠率下降一致[16]。同时,着床位点宫腔没有完全闭合,蜕膜化范围缩小,证实子宫内膜的Mφ的内膜中保守表达的VEGFA可能参与了内膜容受性的构建[17, 18],使内膜血管通透性增强,血管迅速生长,从而血液供应更加丰富,激活胚泡着床[19, 20]。
与肿瘤相关性巨噬细胞在侵袭位点聚集并分泌VEGFA 相似的是,正常妊娠小鼠Mφ数量在着床窗与VEGF的表达呈相关性增长[9]。本研究中也发现在着床位点,巨噬细胞未受影响的实验组右侧、对照组和空白组VEGFA 呈现均一高表达;当抑制着床期子宫内膜Mφ后,实验组左侧注射CL 侧着床点VEGFA的表达量显著减少,胚胎着床率随之降低,提示Mφ可能是通过VEGFA作为下游信号分子,参与构建蜕膜局部血管生成微环境,调控着床过程。同时我们也发现,与巨噬细胞数在着床位点高于非着床位点分布类似的是,着床位点的VEGFA表达与非着床点相比较强,可能参与了着床点的选择,其具体机制还需要进一步研究。
| [1] | Granot I, Gnainsky Y, Dekel N. Endometrial inflammation and effect on implantation improvement and pregnancy outcome[J]. Reproduction, 2012, 144(6): 661-8. ( 1) 1) |
| [2] | Gnainsky Y, Granot I, Aldo P, et al. Biopsy-induced inflammatory conditions improve endometrial receptivity: the mechanism of action[J]. Reproduction, 2015, 149(1): 75-85. ( 1) 1) |
| [3] | Ledee N, Petitbarat M, Chevrier L, et al. The uterine immune profile may help women with repeated unexplained embryo implantation failure after in vitro fertilization[J]. Am J Reprod Immunol, 2016, 75 (3): 388-401. ( 1) 1) |
| [4] | Gong X, Tong Q, Chen Z, et al. Microvascular density and vascular endothelial growth factor and osteopontin expression during the implantation window in a controlled ovarian hyperstimulation rat model [J]. Exp Ther Med, 2015, 9(3): 773-9. ( 1) 1) |
| [5] | Riabov V, Gudima A, Wang N, et al. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis[J]. Front Physiol, 2014, 5: 75. ( 1) 1) |
| [6] | Ribatti D. Mast cells and macrophages exert beneficial and detrimental effects on tumor progression and angiogenesis[J]. Immunol Lett, 2013, 152(2): 83-8. ( 1) 1) |
| [7] | Casazza A, Laoui D, Wenes M, et al. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity[J]. Cancer Cell, 2013, 24(6): 695-709. ( 1) 1) |
| [8] | Liu C, Li Z, Wang L, et al. Activating transcription factor 4 promotes angiogenesis of breast cancer through enhanced macrophage recruitment [J]. Biomed Res Int, 2015, 2015: 974615. ( 1) 1) |
| [9] | Tan W, Chen L, Guo L, et al. Relationship between macrophages in mouse uteri and angiogenesis in endometrium during the periimplantation period[J]. Theriogenology, 2014, 82(7): 1021-7. ( 2) 2) |
| [10] | Gao R, Chen L, Tan W, et al. [Role of macrophages in mouse uterine during the peri-implantation period][J]. Nan Fang Yi Ke Da Xue Xue Bao, 2015, 35(3): 365-9. ( 1) 1) |
| [11] | Care AS, Diener KR, Jasper MJ, et al. Macrophages regulate corpus luteum development during embryo implantation in mice[J]. J Clini Investig, 2013, 123(8): 3472-87. ( 1) 1) |
| [12] | Naito M, Nagai H, Kawano S, et al. Liposomeencapsulated dichloromethylene diphosphonate induces macrophage apoptosis in vivo and in vitro[J]. J Leukoc Biol, 1996, 60(3): 337-44. ( 1) 1) |
| [13] | Gao X, Wang YS, Li XQ, et al. Macrophages promote vasculogenesis of retinal neovasculari-zation in an oxygen-induced retinopathy model in mice[J]. Cell Tissue Res, 2016.( 1) 1) |
| [14] | Ma Y, Li Y, Jiang L, et al. Macrophage depletion reduced brain injury following middle cerebral artery occlusion in mice[J]. J Neuroinflammation, 2016, 13 (1): 38. ( 1) 1) |
| [15] | Van der Hoek K H, Maddocks S, Woodhouse C M, et al. Intrabursal injection of clodronate liposomes causes macrophage depletion and inhibits ovulation in the mouse ovary[J]. Biol Reprod, 2000, 62(4): 1059-66. ( 1) 1) |
| [16] | Pollard JW, Hunt JS, Wiktor-Jedrzejczak W, et al. A pregnancy defect in the osteopetrotic (op/op) mouse demonstrates the requirement for CSF-1 in female fertility[J]. Dev Biol, 1991, 148 (1): 273-83. ( 1) 1) |
| [17] | Allegra A, Marino A, Coffaro F, et al. Is there a uniform basal endometrial gene expression profile during the implantation window in women who became pregnant in a subsequent ICSI cycle?[J]. Hum Reprod, 2009, 24(10): 2549-57. ( 1) 1) |
| [18] | Bourdiec A, Ahmad SF, Lachhab A, et al. Regulation of inflammatory and angiogenesis mediators in a functional model of decidualized endometrial stromal cells[J]. Reprod Biomed Online, 2016, 32(1): 85-95. ( 1) 1) |
| [19] | Liu YX, Gao F, Wei P, et al. Involvement of molecules related to angiogenesis, proteolysis and apoptosis in implantation in rhesus monkey and mouse[J]. Contraception, 2005, 71(4): 249-62. ( 1) 1) |
| [20] | Binder NK, Evans J, Gardner DK, et al. Endometrial signals improve embryo outcome: functional role of vascular endothelial growth factor isoforms on embryo development and implantation in mice[J]. Hum Reprod, 2014, 29(10): 2278-86.( 1) 1) |


