Mutations in CACNA1A cause at least 3 allelic diseases, namely type 2 episodic ataxia (EA2, OMIM #108500), familial hemiplegic migraine type 1 (FHM1, OMIM # 141500) [1, 2], and spinocerebellar ataxia type 6 (SCA6, OMIM #183086) [3]. The CACNA1A gene encodes the P/ Q-type voltage-gated calcium-channel Cav2.1 subunit, which is mainly expressed in the Purkinje and granule cells of the cerebellum [4]. Nonsense and missense mutations of the CACNA1A gene account for most cases of EA2, and large deletions, duplications, rearran- gements, and mutations in its 5' and 3' regions expand the mutation spectrum of EA2 [5, 6, 7]. Mutations in CACNA1A in EA2 result in loss of function of Cav2.1 currents. Most EA2 patients respond well to acetazolamide, a carbonic anhydrase inhibitor, which can prevent the episodic symptoms but cannot improve progressive ataxia [8, 9]. Missense mutations in FHM1 or CAG repeat expansions in CACNA1A are related with increases in Cav2.1 currents. So far no specific me- dicine has been available for FHM1 and SCA6 patients.
There is much clinical overlap among EA2, FHM1 and SCA6. Genetic diagnosis is currently applied by many clinical centers for diagnosis and treatment guidance. Here we reported the identification of a missense mutation in CACNA1A (p.Arg1345Gln) in a Chinese family of ataxia with episodic head and trunk tremor, and the proband did not respond to methazolamide, a carbonic anhydrase inhibitor. We hypothesized that the mutation p.Arg1345Gln in CACNA1A may cause a gain of function of CACNA1A to result in increased calcium entry. After treatment with the calcium channel blocker, flunarizine, the patient's tremor symptom was obviously relieved and well controlled with continuing treatment.
PATIENTS AND METHODS Proband and affected family membersIn October, 2013, a 19-year-old male patient was admitted in our department for uncontrolled body tremor, bilateral lower legs weakness and fatigue for 7 days. The patient reported his first experience of similar symptoms in November, 2011 lasting only for 10 s, for which he did not seek any medical attention. Four months later when he had recurrent episodes of tremor, he was brought to our out-patient clinic and was hospitalized. The episodes were provoked by stress and alleviated after rest. The patient had no difficulty in standing or walking. He recalled that he was neither good at running nor at exercises such as jogging and running since childhood. Physical examinations revealed awkward rotation, inaccurate finger-to-nose test and heel-knee-shin test and wide based stance. Brain computed tomography (CT) scans, magnetic resonance imaging (MRI) and electroencephalography (EEG) were unremarkable. He was diagnosed with ataxia, received no special treatment and recovered in several days. Upon admission this time, which was nearly 2 years later, the patient had more severe tremor symptoms that were precipitated by emotional s tress. He also complained of mild headache. Physical examinations showed head and trunk coarse tremor, intentional tremor, awkward rotation and drunken gait. Romberg test was positive. He was unable to stand when closing eyes.
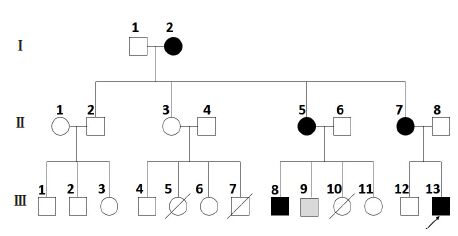
Suspecting that the patient's condition could be of a hereditary nature, we conducted an investigation for similar symptoms among the other members of the patient's family. The results showed that, as indicated in Fig.1, 4 of the patient's family members, the grandmother (72 years old), an aunt (43 years old), mother (40 years old), and a cousin (21 years old), had slight awkward gaits since their childhood. They all had intentional tremor with or without slurred speech. Only the patient's mother reported episodic attack of tremor when she was tired since the age of 26 years, and the symptoms lasted from seconds to minutes, occurring 3 or 4 times per year; and each time the attack occurred, she recovered after rest. Individual Ⅲ-5 died at day 20 after birth and Ⅲ-10 died at day 7 after birth, and their causes of deaths were unclear. Individual Ⅲ-7 died of lung cancer when he was 19 years old.
|
Fig.1 Pedigree of the family with ataxia accompanied by episodic tremor. Ⅰ, Ⅱ, and Ⅲ represent 3 generations. Individuals who had ataxia with or without episodic head and trunk tremor are indicated by a black filled circle (female) or square (male). Arrow indicates the proband (Ⅲ-13), a 19 year-old man. A shadow-filled square (Ⅲ-9) indicates a mutant carrier without any symptom, who was 20 years old. |
To understand the genetic nature of their conditions, we advised the patients identified in this family to receive genetic analysis for gene mutation screening. All the patients or their guardians gave written informed consent for genetic analyses and research, which was approved by the Ethics Committee of Nanfang Hospital, Southern Medical University (Guangzhou, China).
Candidate gene mutation screening for ataxia was performed for the proband. The proband was first screened for spinocerebellar ataxia, SCA1, 2, 3, 6, 7, 12 and DRPLA by CAG repeat expansions. Due to the negative result, next-generation sequencing (NGS) coupled with DNA target-capture array was performed on Immumina HiSeq2000 platform by BGI (Shenzhen, China) as reported [10, 11]. The solid phase array captured all exons, splice sites and the immediately adjacent intron sequences of 1508 genes involved in genetic diseases including 19 ataxia causative genes.
The reported candidate gene (CACNA1A) mutation was further confirmed by Sanger sequencing for all the suspected cases in the family as well as the unaffected family members as controls. The suspected mutation primers were as follows:
CACNA1A-exon25F (forward): 5'-ACCAACCCTGGGAC CAGAAC-3',
CACNA1A-exon25R (reverse): 5'-TACTGCCATCTGCTG GGAAG-3'.
TreatmentThe patient was diagnosed as having EA2 before the result of NGS genetic analysis had been available. Upon this diagnosis, he was treated with one tablet of methazolamide (50 mg) for 3 times per day for one month, but did not show responses. After the identifi- cation of the CACNA1A mutation by genetic diagnosis, the patient received long-term treatment with 2 flunari- zine tablets (5 mg/tablet) in the morning and one in the afternoon till now. By now the patient has been followed up for 3 years. No drug was given to the other affected members of his family because of their mild symptoms.
RESULTS Results of genetic analysisGenetic analysis of the proband for screening spinocerebellar ataxia, SCA1, 2, 3, 6, 7, 12 and DRPLA did not yield unremarkable findings. Further DNA analysis of the proband with NGS coupled with target-capture array identified a known pathologic heterozygous missense mutation in CACNA1A (NM_ 001127221.1, c.4034G-<A, p.Arg1345Gln, exon 25). This mutation was reported in a Portuguese family with slowly progressive ataxia and hemiplegic migraine, causing an arginine-to-glutamine change to result in gain of function of Cav2.1 currents [12].
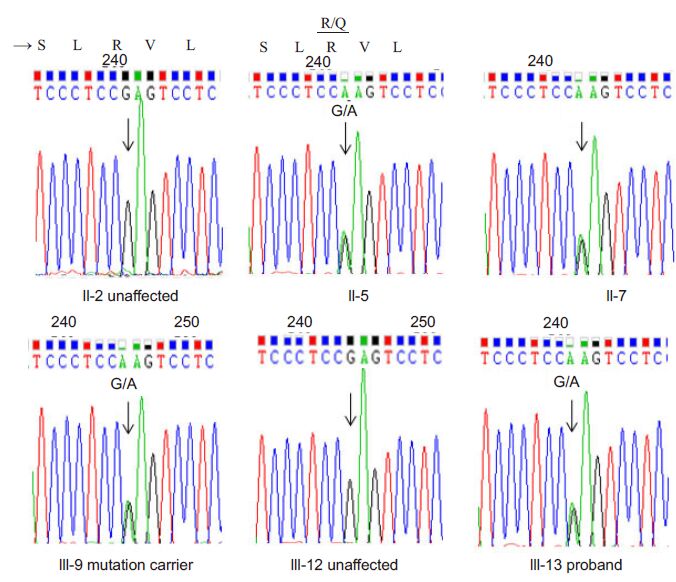
All the DNA samples from generations Ⅱ and Ⅲ of the pedigree were analyzed. As illustrated in Fig.2, Sanger sequencing confirmed this mutation in the proband and all the other 4 affected members and in a 20-year-old carrier without symptoms yet. This result demonstrates that mutation in CACNA1A (p. Arg1345Gln) is the pathologic mutation for this family.
Treatment outcomesThe patient's symptoms of tremor persisted while he was waiting for genetic results. During this period, the patient was unable to work. Given that the patient was not responsive to methazolamide and R1345Q mutation may cause an excess of intracellular calcium entry as reported in the Portuguese family study [12], we treated the patient with calcium channel blocker (CCB), flunarizine (5 mg/tablet, two tablets in the morning and one tablet in the afternoon). Because calcium channel blockers have been standard migraine prophylactic drugs for all FHM [13], p.R1345Q mutation was described in a patient with ataxia and migraine, and our patient had mild headache when he had attack of tremor, informed consent was taken for flunarizine treatment. We did not apply for clinical trial registration. The patient's tremor resolved gradually after 3-4 days of treatment except for ataxia. His symptoms recurred once he stopped the medication during the 3-year follow-up.
|
Fig.2 Identification a heterozygous mutation in CACNA1A gene (NM_ 001127221.1, c.4034G-<A, p. Arg1345Gln, exon 25). Sanger sequencing for the exon 25 of CACNA1A was performed with DNA samples from all Ⅱ and Ⅲ generations. Examples of normal or mutation in c.4034G-<A of CACNA1A are illustrated. Encoded amino acids are represented as one character at the top of 3 nucleotide sequences. Arrows indicate the G→A heterozygous mutation. |
The clinical features (without hemiplegic migraine) and treatment outcomes of the patient suggested that our case did not match the diagnosis of EA2, FHM1, or SCA 6, but probably represented a new type of ataxia with episodic tremor. The patient still had ataxia, but was free of recurrent attack of tremor, and was able to work. The treatment result suggests that R1345Q mutation in CACNA1A may cause gain of function of Cav2.1 currents, which explains the patient's unresponsiveness to methazolamide treatment.
DISCUSSIONHere we presented a Chinese family having ataxia with or without recurrent attack of tremor. Genetic analysis revealed a missense mutation R1345Q in CACNA1A as the disease-causing mutation. The patient responded to flunarizine but not to methazolamide.
R1345Q mutation in CACNA1A identified in our case was previously reported in a Portuguese family with progressive ataxia and hemiplegic migraine (R1347Q) [12]. Different from the Portuguese case, our patient mainly suffered recurrent attacks of head and trunk tremors in addition to intention tremor. Reports had docu- mented one case of intention tremor caused by T666M mutation [14] and two cases with head tremor caused by mutation of either p.Cys1370Tyr [15] or p.Leu617Val [16]. Therefore, to the best of our knowledge, there is no previous case report of patients carrying a CACNA1A mutation and exhibiting head and trunk tremor as the most significant presenting symptom. Hemiplegic migraine did not occur in this present case, and all the other affected family members had only mild ataxia; imaging examinations revealed no remarkable brain atrophy in our case, which was present in most of the affected Portuguese family members. We thus conclude that the same mutation in CACNA1A gene may cause different phenotypes in the two families.
The patient did not respond to carbonic anhydrase inhibitor, which is currently the most efficient treatment for EA2 patients. In a case report of CACNA1A mutation that was associated with paroxysmal head tremor, the patient responded well to acetazolamide [16]. Alonso et al [12] suggested that R1345Q mutation in the S4- transmembrane segments domain Ⅲ might have gain of function of the calcium channel because it was similar to R192Q and R583Q mutations located in the S4-transmembrane segments domains Ⅰ and Ⅱ, which increase the calcium currents [12, 17, 18]. However, there is no experimental evidence to support this assumption, nor did the authors reporting the Portuguese familial case described treatment of the affected members [12]. We hypothesize that R1345Q mutation in our case might also increase the function of the channel to result in an excess of intracellular calcium. Treatment of the patient with the calcium channel blocker flunarizine effectively relieved the symptoms. Flunarizine treatment provides a new strategy for patients who carry a CACNA1A mutation with gain of function of calcium fluxes. Based on the clinical features and treatment effect, we presume that our case may not belong to EA2, FHM1, or SCA 6, but represents a new type of ataxia with episodic tremor. Additional experimental evidences are required to verify any functional ch anges related with R1345Q mutation, and the effect of the flunarizine treatment awaits further confirmation by large-scale clinical trials.
In conclusion, we believe that this ataxia family with or without episodic attacks of tremor expands the phenotype of CACNA1A mutations. Genetic diagnosis has provided important guidance for our decision on flunarizine treatment for the patient.
AcknowledgementsWe are grateful to our patient and his family for participating in this study. We also thank Dr. Binukumar BK at NINDS/NIH for proofreading this manuscript.
| [1] | Ophoff RA, Terwindt GM, Vergouwe MN, et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4[J]. Cell, 1996, 87(3): 543-52. ( 1) 1) |
| [2] | Riess O, Schols L, Bottger H, et al. SCA6 is caused by moderate CAG expansion in the alpha1A-voltage-dependent calcium channel gene[J]. Hum Mol Genet, 1997, 6(8): 1289-93. ( 1) 1) |
| [3] | Zhuchenko O, Bailey J, Bonnen P, et al. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel[J]. Nat Genet, 1997, 15(1): 62-9. ( 1) 1) |
| [4] | Rajakulendran S, Schorge S, Kullmann DM, et al. Dysfunction of the Ca(V)2.1 calcium channel in cerebellar ataxias[J]. F1000 Biol Rep, 2010, 2: 1-4.( 1) 1) |
| [5] | Riant F, Mourtada R, Saugier-Veber P, et al. Large CACNA1A deletion in a family with episodic ataxia type 2[J]. Arch Neurol, 2008, 65(6): 817-20. ( 1) 1) |
| [6] | Veneziano L, Guida S, Mantuano E, et al. Newly characterised 5' and 3' regions of CACNA1A gene harbour mutations associated with familial hemiplegic migraine and episodic ataxia[J]. J Neurol Sci, 2009, 276(1-2): 31-7. ( 1) 1) |
| [7] | Labrum RW, Rajakulendran S, Graves TD, et al. Large scale calcium channel gene rearrangements in episodic ataxia and hemiplegic migraine: implications for diagnostic testing[J]. J Med Genet, 2009, 46(11): 786-91. ( 1) 1) |
| [8] | Griggs RC, Moxley RT, Lafrance RA et al. Hereditary paroxysmal ataxia: response to acetazolamide[J]. Neurology.1978, 28(12): 1259-64. ( 1) 1) |
| [9] | Jen JC, Graves TD, Hess EJ, et al. Primary episodic ataxias: diagnosis, pathogenesis and treatment[J]. Brain, 2007, 130(Pt 10): 2484-93. ( 1) 1) |
| [10] | Wei X, Ju X, Yi X, et al. Identification of sequence variants in genetic disease-causing genes using targeted next-generation sequencing[J]. PloS ONE, 2011, 6(12): e29500. doi: 10.1371/journal. pone.0029500. ( 1) 1) |
| [11] | Hu Y, Jiang H, Wang Q, et al. Identification of a novel nonsense mutation p.Tyr1957Ter of CACNA1A in a Chinese family with episodic ataxia 2[J]. PloS ONE, 2013, 8(2): e56362. doi: 10.1371/ journal.pone.0056362.( 1) 1) |
| [12] | Alonso I, Barros J, Tuna A, et al. A novel R1347Q mutation in the predicted voltage sensor segment of the P/Q-type calcium-channel alpha-subunit in a family with progressive cerebellar ataxia and hemiplegic migraine[J]. Clin Genet, 2004, 65(1): 70-2. ( 6) 6) |
| [13] | Jen JC. Familial Hemiplegic Migraine. In: Pagon RA, Adam MP, Ardinger HH, et al, editors [OL]. GeneReviews(R). Seattle (WA) 1993. (http://www.ncbi.nlm.nih.gov/pubmed/20301562,2016/6/16). ( 1) 1) |
| [14] | Dichgans M, Herzog J, Freilinger T, et al. 1H-MRS alterations in the cerebellum of patients with familial hemiplegic migraine type 1[J]. Neurology, 2005, 64(4): 608-13. ( 1) 1) |
| [15] | Geerlings RP, Koehler PJ, Haane DY, et al. Head tremor related to CACNA1A mutations[J]. Cephalalgia, 2011, 31(12): 1315-9. ( 1) 1) |
| [16] | Molloy A, Kimmich O, Martindale J, et al. A novel CACNA1A mutation associated with adult-onset, paroxysmal head tremor[J]. Mov Disord, 2013, 28(6): 842-3. ( 2) 2) |
| [17] | Hans M, Luvisetto S, Williams ME, et al. Functional consequences of mutations in the human alpha1A calcium channel subunit linked to familial hemiplegic migraine[J]. J Neurol Sci, 1999, 19(5): 1610-9. ( 1) 1) |
| [18] | Kraus RL, Sinnegger MJ, Koschak A, et al. Three new familial hemiplegic migraine mutants affect P/Q-type Ca2+ channel kinetics [J]. J Biol Chem, 2000, 275(13): 9239-43.( 1) 1) |


