2. General Hospital of PLA, Beijing 100853, China ;
3. Institute of Aviation Medicine, Beijing 100142, China
2. 解放军总医院,北京 100853 ;
3. 航空医学研究所,北京 100142
Polyphenol compounds with unique chemical structures have been shown to scavenge reactive radical species and bind to metal ions to prevent peroxidative stress[1]and are capable of preventing damages of lipid membrane, proteins and nucleic acids induced by reactive oxygen species (ROS) and nitric oxide[2-3]. In both in vitro and in vivo models, polyphenols are found to possess anti-carcinogenic activity, inhibit the activity of many transcription factors, and suppress the growth of human cancer cells [4-6]. Accumulating evidence also support the cardioprotective effects of tea polyphenols (TP) against ischemia-reperfusion (IR) injury[7-11], and in different animal models, treatment with tea extract or catechins before or during IR of the heart was found to improve the cardiac function, reduce the infarct size, and ameliorate apoptosis of the cardiac myocytes [7-11]. But so far, the mechanisms underlying the cardioprotective action of TP have not been fully understood.
Canyon et al [12]reported that intravenous infusion of adenosine and lidocaine solution protected against severe arrhythmias, reduced infarct size and prolonged the survival of rats with regional ischemia probably in association with preserving myocardial high-energy phosphates, lowering myocardial metabolic demand at the expense of a high acid-load during ischemia, and allowing for a rapid recovery of myocardial pH during reperfusion. Research evidence suggests that an increased myocardial cytoplasmic free calcium concentration is involved in burn injury-induced suppression of myocardial contractile function in rats[13], and the selective inhibition of Na2+/Ca2+ exchanger may effectively preserve high-energy phosphates and improve cardiac function after reperfusion in Langendorffperfused rat hearts [14]. Earlier studies showed that inhibition of xanthine oxidase by allopurinol may protect the heart from IR injury by enhancing energy supply, and histidine prevented postischemic reperfusion injury in isolated heart by inhibiting ROS and reserving high-energy phosphates[15, 16].
Considering the importance of energy metabolism and cellular calcium homeostasis in myocardial function and the close relation of high-energy phosphates with the protective effects of some antixodiants against IR injury, we hypothesized that TP offers protection against heart IR injury by influencing myocardial energy metabolism and calcium inward current. To test this hypothesis, we conducted this study by measuring energy metabolism in Langendorff-perfused rat hearts with IR injury and by detecting calcium inward current (ICa-L) in cultured rat cardiac myocytes.
MATERIALS AND METHODS AnimalsTwelve 12-week-old Wistar rats of either sex (weighing 300 to 350 g) were maintained in 6 separate cages (2 rats in each cage) in a controlled standard environment at 22℃with a 12-hour dark/light cycle. The rats were handled with humane care in line with the guidelines of the Chinese Council on Animal Care and the Research Committee on Animal Care and Supply. The rats were acclimated to the conditions for 3 days and were subsequently assigned to control (n=6) and IR (n=6) groups. All the rats were allowed free access to normal chow and water for 7 days until the time of the surgery.
Chemical agentsTP with a purity >97% (extracted from tea using a modified method of Yang et al[17]) was manufactured and provided by Tiantai Pharmaceutical Factory (Zhejiang, China). According to the manufacturer, this product contained (-)-epigallocatechin gallate (EGCG) (50%-60%), (-)-epicatechin gallate (ECG) (15%-20%), (-)-epigallocatechin (EGC) (10%-15%), (-)-epicatecthin (EC) (4%-6%), and (+)-catecthin (C) (2%-4%). In this experiment, TP was dissolved in modified KrebsHenseleit (K-H) solution for Langendroff-perfused rat hearts or for treatment of cultured rat cardiac myocytes. All the other reagents were of analytical grade.
Measurement of myocardial function in Langendorff rat heart with IR injury
The Langendorff heart was rapidly excised from Wistar rat and perfused with a modified K-H solution (containing KCl 5.4 mmol/L, MgSO4·7H2O 0.8 mmol/L, CaCl2·H2O 2.2 mmol/L, NaCl 9.2 mmol/L, EDTA 0.5 mmol/L, and glucose 11 mmol/L) according to our previous method[18]. The perfusion solution was gassed with 95% O2 and 5% CO2 and maintained at 37.4℃with a pH value of 7.4 (adjusted by controlling the flow of oxygen and carbon dioxide). The hearts in IP-treated group were perfused with K-H solution containing 2.5 mg/L TP. The hearts were exposed to 30 min of global ischemia at 18℃followed by reperfusion for 30 min. A latex balloon was introduced into the left ventricle via the left atrium and connected to a transducer (P-0.5A, Japan) for measurement of the physiological indices of the Langendorff heart before the ischemia, after the 30-min ischemia, and at 5, 15, or 30 min during the reperfusion.Left ventricular developed pressure (LVDP), +dp/dtmax and-dp/dtmax in the control and TP groups were measured and analyzed using CARDIO2 software developed by Shanghai Medical University (Shanghai, China).
Determination of myocardial energy metabolism in Langendorff rat heart with IR injuryThe heart connected to the Langendorff perfusion apparatus was placed in a standard 20-mm nuclear magnetic resonance (NMR) tube with the apex approximately 2.5 cm from the bottom of the tube, and the tube was inserted into the NMR coil. Using 31P-NMR technique following the protocols described by Cheng et al[19], the myocardial energy metabolism in Langendorff rat heart was measured before ischemia, at 5, 15, and 30 min during ischemia, and at 5, 15, or 30 min during reperfusion. The phosphorous nuclear resonance frequency was 61.83 MHz. The NMR spectra were collected using signal pulse for 150 scans in about 5 min, so that the signals obtained in each spectrum should represent an accumulated value during the acquisition time. ATP and PCr were quantified by comparison with a capillary tube of standard methylenediphosphonic acid (MDP, 0.25 mol/L) fixed inside the NMR tube. Phosphate peaks, expressed as percentages of the control values, were determined by measuring the area under each resonance peak. The relative intensity of each peak was used for quantitative analysis. The data obtained were fitted to chemical-shift expression.
Determination of calcium inward current in cultured rat cardiomyocytesA single cardiomyocyte from the left ventricle of adult Wistar rat heart was enzymatically isolated by Langendorff perfusion of the aorta[20]. Briefly, the heart was rapidly removed and placed in oxygenated ice-cold Ca2+-free Tyrode's solution; the Langendorff heart was treated by a 5 min perfusion with a nominally Ca2+-free Tyrode's solution. Enzymatic digestion was initiated by 15-20 min of perfusion with 50 mL Ca2+-free Tyrode's solution containing 15 mg collagenase. At the end of the perfusion, the ventricles were excised and cut into small pieces, which were stirred in a small vessel containing Kraft-ebrühe (KB) solution until elongated, striated cardiomyocytes dissociated from the tissue blocks. Cardiomyocytes were harvested after filtering the cell-containing suspension through a nylon mesh (pore size 200μm), washed for 3 times with the storage solution and then maintained at room temperature in KB solution for at least 1 h before the electrophysiological experiment. The cardiomyocytes were placed in a chamber mounted on an inverted microscope (Olympus Inc. Tokyo, Japan). Glass microelectrodes (1-1.5μm in diameter) were pulled with a horizontal puller. The cardiomyocytes were treated with gravity feeding TP at the concentration of 2.5 or 5.0 mg/L for 3 min. Before and after the application of TP, the control and treated cell ICa-L values were recorded using whole cell patch-clamp technique. After gigaseal was formed and the patch ruptured, an Axopatch-700B patch clamp amplifier (Axon Instruments, USA) was used for voltage clamping, and ICa-L was obtained by voltage clamp steps of 250-ms duration from a-80 mV holding potential to the test potentials between-40 and + 40 mV. During current measurements, the cell capacitance and series resistance were compensated, and pCLAMP 9.2 software package (Axon) was used for data acquisition and analysis.
Statistical analysesThe data are presented as Mean±SD. Statistical analyses were performed with one-way analysis of variance, and a P value < 0.05 detected by F-test was considered to indicate a significant difference between the groups.
RESULTS Effect of TP on myocardial function in Langendorff rat heart with IR injuryBefore reperfusion, the Langendorff rat hearts were arrested for 30 min at 18℃. At the end of the 30-min reperfusion, LVDP, + dp/dtmax, -dp/dtmax and CF in TP group were significantly higher than those in the control group (P < 0.05, Tab. 1).
| Table 1 Effect ofTP on myocardial function in Langendorff rat heart with IR injury (n=6, Mean±SD) |
In the control hearts, PCr declined rapidly and became undetectable after 15 min of ischemia, at which point PCr level maintained 6.4% of the pre-ischemic level in TP group (P < 0.01, Tab. 2). PCr showed a significantly greater recovery during reperfusion in TP group than in the control group (P < 0.05). ATP decreased more slowly in TP group than in the control group during the ischemic phase, and in the reperfusion phase and thereafter, ATP level was significantly higher in TP group than in the control group (P < 0.05, Tab. 2).
| Table 2 Effect of TP on myocardial energy metabolism in Langendorff rat heart with IR injury (n=6, Mean±SD) |
The action potential duration of repolarization (APD50 and APD90) of rat myocytes was shortened by treatment with TP. The density of peak ICa-L was significantly lowered by TP, and pA/pF was reduced from 12.5±1.2 to 8.6±1.5 in cells treated with 2.5 mg/L TP, and from 11.1±0.9 to 5.4±0.5 in cells treated with 5.0 mg/L TP (P < 0.01, Fig. 1).
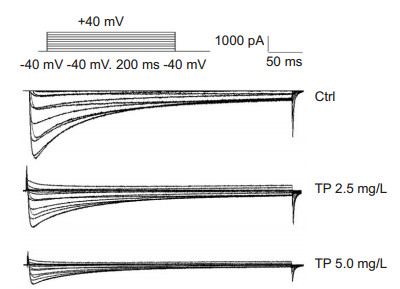
|
Figure 1 Effect of different concentrations of TP on ICa-L in isolated rat myocytes (n=5). |
Many investigations have shown the protective effects of TP or its main composites against cardiac IR injury both in vivo and in vitro. Yanagi et al[21]reported that pre-treatment of rats with green tea polyphenol orally at 0.1, 1, or 10 mmol/L for 2 weeks preserved cardiac function of Langendorff-perfused hearts with global IR stress, and this effect may due to the antioxidative and antiapoptotic activities of polyphenols. Akhlaghi et al[22] observed that rats fed with green tea showed significantly decreased markers of apoptosis, increased total glutathione, and enhanced activities of the phase 2 enzymes glutamate cysteine ligase and quinone reductase in the hearts with IR stress. Hirai et al[10] examined the protective effects of (-)-epigallocatechin gallate (EGCG) or gallocatechin gallate GCG against IR injury in perfused guinea pig Langendorff hearts and found that both EGCG (3×10-5 mol/L) and GCG (3×10-6 mol/L) significantly promoted the recovery of LVDP from IR stress, which was consistent with a significant increase of ATP generation in tissues after ischemia and reperfusion. Our findings are consistent with these observations.
The cardioprotective effects of antioxidants on perfused hearts are thought to be mediated by the inhibition of Na2+/Ca2+ exchanger that protects the mitochondrial respiratory function and reduces lipid peroxidative injury [14-16]. Iwai et al [23] observed that cytosolic sodium overload may induce mitochondrial dysfunction in cardiac cells during ischemia and resulted subsequently in post-ischemic contractile dysfunction in perfused rat hearts. The cardioprotection of diltiazem may be exerted via attenuating cytosolic Na+ overload through inhibition of Na2+ channels in the isc hemic heart and preservation of mitochondrial functions during ischemia to improve post-ischemic energy production and promote contractile recovery [24]. KR-32570 possessed potent cardioprotective effects in perfused rat hearts by inhibiting Na2+/H+ exchanger and lipid peroxidation and preserving high-energy phosphates [25]. Hirai et al [10]observed that in perfused rat hearts, EGCG (10-5 mol/L) inhibited mitochondrial Ca2+ elevation by lowering Ca2+ content or suppressing acidi fication of perfusate. In this study and also a previous study[26], we found that TP significantly inhibited ICa-L in cardiac myocytes in vitro, suggesting the beneficial effect of TP in maintaining normal mitochondrial function in rat hearts exposed to IR injury. In this study, we further demonstrated the protective effects of TP on myocardial function in isolated rat heart with IR injury. Our findings support a possible role of TP in maintaining myocardial energy metabolism and in inhibition of calcium inward current caused by myocardial IR injury, but the mechanism still awaits further study.
Acknowledgment: The authors wish to thank Mr. CHENG Zengjiang for his assistance in the experiment.| [1] |
Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance[J].
Nutr Rev,1998, 56 : 317-33.
( 0) 0)
|
| [2] |
Aviram M, Dornfeld L, Kaplan M, et al. Pomegranate juice flavonoids inhibit low-density lipoprotein oxidation and cardiovascular diseases: studies in atherosclerotic mice and in humans[J].
Drugs Exp Clin Res,2002, 28 : 49-62.
( 0) 0)
|
| [3] |
Leenen R, Roodenburg AJ, Tijburg LB, et al. A single dose of tea with or without milk increases plasma antioxidant activity in humans[J].
Eur J Clin Nutr,2000, 54 : 87-92.
DOI: 10.1038/sj.ejcn.1600900. ( 0) 0)
|
| [4] |
Lambert JD, Yang CS. Cancer chemopreventive activity and bioavailability of tea and tea polyphenols[J].
Mutat Res,2003, 523-4 : 201-208.
( 0) 0)
|
| [5] |
Naasani I, Oh-Hashi F, Feng WY, et al. Blocking telomerase by dietary polyphenols is a major mechanism for limiting the growth of human cancer cells in vitro and in vivo[J].
Cancer Res,2003, 63 : 824-30.
( 0) 0)
|
| [6] |
Ahmad N, Gupta S, Mukhtar H, et al. Green tea polyphenol epigallocatechin-3-gallate differentially modulates nuclear factor kappa B in cancer cells versus normal cells[J].
Arch Biochem Biophys,2000, 376 : 338-46.
DOI: 10.1006/abbi.2000.1742. ( 0) 0)
|
| [7] |
Potenza MA, Marasciulo FL, Tarquinio M, et al. EGCG, a green tea polyphenol, improves endothelial function and insulin sensitivity, reduces blood pressure, and protects against myocardial I/R injury in SHR[J].
Am J Physiol Endocrinol Metab,2007, 292 : E378-87.
( 0) 0)
|
| [8] |
Yamazaki KG, Romero-Perez D, Barraza-Hidalgo M, et al. Short-and long-term effects of (-)-epicatechin on myocardial ischemiareperfusion injury[J].
Am J Physiol Heart Circ Physiol,2008, 295 : H761-7.
DOI: 10.1152/ajpheart.00413.2008. ( 0) 0)
|
| [9] |
Modun D, Music I, Katalinic V, et al. Comparison of protective effects of catechin applied in vitro and in vivo on ischemia reperfusion injury in the isolated rat hearts[J].
Croat Med J,2003, 44 : 690-6.
( 0) 0)
|
| [10] |
Hirai M, Hotta Y, Ishikawa N, et al. Protective effects of EGCg or GCg, a green tea catechin epimer, against postischemic myocardial dysfunction in guinea-pig hearts[J].
Life Sci,2007, 80 : 1020-32.
DOI: 10.1016/j.lfs.2006.11.032. ( 0) 0)
|
| [11] |
Townsend PA, Scarabelli TM, Pasini E, et al. Epigallocatechin-3-gallate inhibits STAT-1 activation and protects cardiac myocytes from ischemia/reperfusion-induced apoptosis[J].
FASEB J,2004, 18 : 1621-3.
( 0) 0)
|
| [12] |
Canyon SJ, Dobson GP. The effect of an adenosine and lidocaine intravenous infusion on myocardial high-energy phosphates and pH during regional ischemia in the rat model in vivo[J].
Can J Physiol Pharmacol,2006, 84 : 903-12.
DOI: 10.1139/Y06-035. ( 0) 0)
|
| [13] |
Xia Z, Tian J, Tang H, et al. Study on the myocardial contractile function and intracellular free calcium in scalded rats[J].
Chin J Burn,2001, 17 : 342-4.
( 0) 0)
|
| [14] |
Feng NC, Satoh H, Urushida T, et al. A selective inhibitor of Na+/Ca2+ exchanger, SEA0400, preserves cardiac function and high-energy phosphatesagainst ischemia/reperfusion injury[J].
J Cardiovasc Pharmacol,2006, 47 : 263-70.
DOI: 10.1097/01.fjc.0000202561.69291.ac. ( 0) 0)
|
| [15] |
Pisarenko OI, Lakomkin VL, Studneva IM, et al. Allopurinol enhanced postischemic recovery in the isolated rat heart involves repletion of high-energyphosphates[J].
Biochem Med Metab Biol,1994, 51 : 16-26.
DOI: 10.1006/bmmb.1994.1002. ( 0) 0)
|
| [16] |
Cai Q, Takemura G, Ashraf M. Antioxidative properties of histidine and its effect on myocardial injury during ischemia/reperfusion in isolated rat heart[J].
J Cardiovasc Pharmacol,1995, 25 : 147-55.
DOI: 10.1097/00005344-199501000-00023. ( 0) 0)
|
| [17] |
Yang XQ, Ye LY, Jia XS. Extract of tea natural antioxidant[J].
Acta Agric Univ Zhejiang,1992, 18 : 19-22.
( 0) 0)
|
| [18] |
Dong HJ, Hu DH. Effect of xylazine on isolated working heart in rat[J].
Bull Acad Mil Med Sci,1996, 20 : 91-3.
( 0) 0)
|
| [19] |
Cheng ZJ, Du ZH, Li HX, et al. Simultaneous observation of parameter of intraventricular pressure and energy metabolism in rat hearts using nuclear magnetic resonance[J].
Acta Physioloica Sinica,1999, 51 : 700-4.
( 0) 0)
|
| [20] |
Isenberg G, Klockner U. Calcium tolerant ventricular myocytes prepare by preincubation in a"KB medium"[J].
Pflugers Arch,1982, 395 : 6-18.
DOI: 10.1007/BF00584963. ( 0) 0)
|
| [21] |
Yanagi S, Matsumura K, Marui A, et al. Oral pretreatment with a green tea polyphenol for cardioprotection against ischemia reperfusion injury in an isolated rat heart model[J].
J Thorac Cardiovasc Surg,2011, 141 : 511-7.
DOI: 10.1016/j.jtcvs.2010.04.016. ( 0) 0)
|
| [22] |
Akhlaghi M, Bandy B. Dietary green tea extract increases phase 2 enzyme activities in protecting against myocardial ischemia reperfusion[J].
Nutr Res,2010, 30 : 32-9.
DOI: 10.1016/j.nutres.2009.11.002. ( 0) 0)
|
| [23] |
Iwai T, Tanonaka K, Inoue R, et al. Mitochondrial damage during ischemia determines post-ischemic contractile dysfunction in perfused rat heart[J].
J Mol Cell Cardiol,2002, 34 : 725-38.
DOI: 10.1006/jmcc.2002.2002. ( 0) 0)
|
| [24] |
Takeo S, Tanonaka K, Iwai T, et al. Preservation of mitochondrial function during ischemia as a possible mechanism for cardioprotection of diltiazem against ischemia/reperfusion injury[J].
Biochem Pharmacol,2004, 67 : 565-74.
DOI: 10.1016/j.bcp.2003.09.016. ( 0) 0)
|
| [25] |
Lee BH, Seo HW, Yi KY, et al. Effects of KR-32570, a new Na +/H + exchanger inhibitor, on functional and metabolic impairments produced by global ischemia and reperfusion in the perfused rat heart[J].
Eur J Pharmacol,2005, 511 : 175-82.
DOI: 10.1016/j.ejphar.2005.01.045. ( 0) 0)
|
| [26] |
Chen Y, Liu QH. Effects of tea polyphenols on calcium electricity and action potential in guinea pig myocardial cells[J].
Chin Pharm J,2010, 45 : 192-6.
( 0) 0)
|
 2016, Vol. 36
2016, Vol. 36
