2. Outpatient Department, Zhujiang Hospital, Southern Medical University, Guangzhou 510280, China
2. 南方医科大学珠江医院 急诊科, 广东 广州 510280
Chronic kidney disease(CKD) is associated with an increased risk of mortality, cardiovascular events, hospitalization and increased healthcare costs [1-3]. Patients with CKD often have dyslipidemia[4-6], which plays an important role in the progression of kidney disease [6-8]. Statins, inhibitors of 3-hydroxy-3-methylglutaryl CoA reductase that is essential for cholesterol synthesis, are not widely used in patients with CKD[5]. Accumulating evidence from a variety of animal models suggests a role of statins in delaying the progression of kidney disease[9-12], which is attributed to their pleiotropic effects independent of their lipid-lowering effects[9, 13-15]. But so far the effect of statins on the progression of CKD remains controversial. Previous meta-analyses of randomized trials indicated that statin therapy modestly reduced proteinuria and the rate of kidney function loss[16]with a renoprotective effect in CKD patients [17, 18]. According to a Cochrane systematic review, statin use can improve cardiovascular outcomes and prevent future cardiovascular events, but have uncertain effects on the progression of kidney disease [19]. The recent Study of Heart and Renal Protection (SHARP) trial of a wide range of patients with CKD showed that lowering low-density lipoprotein cholesterol (LDL-C) with daily simvastatin (20 mg) plus ezetimibe (10 mg) did not slow kidney disease progression within 5 years [20]. Given these divergent data, we performed a meta-analysis to evaluate whether statins can slow kidney disease progression in patients with CKD.
METHODS Data sources and search strategyWe searched PubMed, EMBASE, Medline, and the Cochrane Central Register of Controlled Trials (CENTRAL) for relevant studies published in English by February, 2015 using following terms: " kidney disease" , " kidney failure" , " chronic kidney disease" , " CKD" , " chronic renal failure" , " dyslipidemia" , " hypercholesterolemia" , " hyperlipidemia" , " HMG-CoA reductase inhibitors" , " HMG-CoA reductase inhibitor" and " statins" . Unpublished studies were also searched in the reference lists of the retrieved articles, in conference proceedings and at https://clinicaltrials.gov/.
Study selectionWe included all the RCTs and quasi-RCTs with a duration of observation for at least 3 months in adult patients with CKD, including subgroup studies of adult patients with CKD recruited within large-scale statin trials. We also included studies that compared statins with placebo, non-statin treatment and routine care (such as low-fat diet therapy). Studies that compared two different statins, different dose of one satin, or a statin with a non-statin regime (including fibrate therapy) were excluded. We defined CKD according to the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines[21]. We also excluded studies that included children with CKD, adult patients on dialysis (peritoneal dialysis or hemodialysis), and kidney transplant recipients. The outcomes of interest were end-stage renal disease (ESRD), doubling of serum creatinine level, a reduction of estimated glomerular filtration rate (eGFR) by over 25%, and changes of eGFR from the baseline at the end of follow-up. As creatinine clearance is commonly used as an estimate of GFR in spite of their conceptual differences[21], we used creatinine clearance interchangeably with GFR to assess the outcomes of the patients.
Data extraction and study quality assessmentTwo reviewers independently screened the titles and abstracts of the potentially eligible articles, and subsequently reviewed the full texts of these articles. We extracted the data of the study sample, participant characteristics, interventions, and outcomes. Where more than one publication of one study were found, we treated it as one study and combined these reports and extracted the complete data. Any disagreements were resolved by consensus. Two authors independently assessed the risk of bias in the included studies using the risk of bias assessment tool [22] and examined the sequence generation, allocation concealment, blinding of investigators, participants, and outcome assessors, completeness of the outcome data, use of intention-to-treat analysis, selective reporting, and other bias.
Data synthesis and analysisWe used relative risk ratios (RR) with 95% confidence interval (CI) to assess the results of dichotomous outcomes (ESRD events, doubling of serum creatinine level, and an eGFR reduction by over 25%). For continuous outcomes (changes in eGFR from baseline at the end of follow-up), we calculated the standardized mean differences (SMD) with 95% CI, where different scales were used (scales of reported eGFR included mL/min, mL/min/1.73 m2, and mL/min/ 1.73 m2/year). When standard deviations (SDs) were unavailable and only the baseline and follow-up eGFR was available, the missing SDs and changes in eGFR from baseline to follow-up were obtained according to the Cochrane Handbook [22]. We used the random effects model to pool the data because of the differences among studies, particularly in such clinical characteristics as statin types and doses, clinical populations, and duration of follow-up. We used the Cochran Q test and I2 test to assess heterogeneity. A P value < 0.05 was considered to indicate a significant difference and I2 values of 25%, 50% and 75% indicated low, medium and high levels of heterogeneity, respectively[23].
We conducted subgroup analyses to explore the effects of baseline eGFR, statin types, and statin intensity on the renal parameters. We defined mild CKD as a baseline eGFR≥60 mL/min/1.73 m2, moderate CKD as a baseline eGFR of 30-59 mL/min/1.73 m2, and severe CKD as a baseline eGFR of < 30 mL/min/1.73 m2. The intensity of statin therapy was categorized as high-intensity, moderate-intensity, and low-intensity according to the definitions from the recent American College of Cardiology/American Heart Association guidelines [24]. High-intensity statin therapy was defined as one with a therapeutic target of lowering LDL-C by 50% (using atorvastatin at 40-80 mg or rosuvastatin at 20-40 mg), moderate-intensity statin therapy as a decrease in LDL-C by 30% to 50% (using atorvastatin at 10-20 mg, rosuvastatin at 5-10 mg, simvastatin at 20-40 mg, fluvastatin XL at 80 mg, fluvastatin at 40 mg bid, pravastatin at 40-80 mg, lovastatin at 40 mg, or pitavastatin at 2-4 mg), and low-intensity statin therapy as lowering LDL-C by approximately < 30% (using simvastatin at 10 mg, fluvastatin at 20-40 mg, pravastatin at 10-20 mg, lovastatin at 20 mg, or pitavastatin at 1 mg). A funnel plot was constructed to assess publication bias. Sensitivity analysis was performed to assess the overall effects of statins by including only those studies that reported blinding of the participants, investigators, and outcome assessors. In additional sensitivity analysis, we excluded studies with a follow-up less than 1 year.
RUSULTS Study selectionFig. 1 shows the flow chart for literature search and selection of articles. We retrieved 129 potentially eligible articles for further assessment, of which 30 RCTs (45 688 participants) of 28 studies [20, 25-51] met our inclusion criteria. Three studies provided data on ESRD, 2 studies provided data on doubling the serum creatinine level, 4 studies including 6 RCTs (one study including 3 RCTs [34]) provided data on an eGFR reduction by over 25%, and 24 studies reported changes in eGFR. The investigated statins included simvastatin (6 studies), pravastatin (8 studies), fluvastatin (3 studies each), atorvastatin (6 studies), cerivastatin (1 study), and lovastatin (1 study).
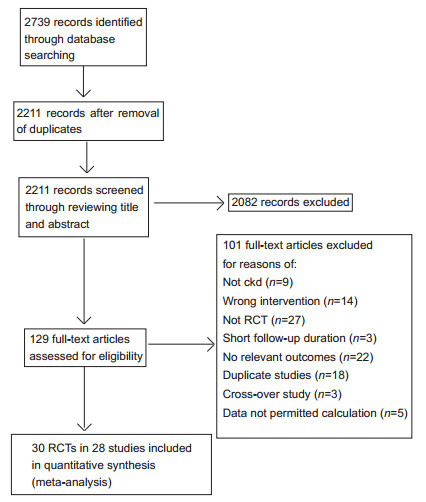
|
Figure 1 Flow chart of literature search and selection. |
The characteristics of the included studies are shown in Tab. 1. The follow-up ranged from 3 months to 6 years. The mean age of the included participants was 58.4 years and 67.4% of the participants were men. The included studies showed a high risk of bias (Fig. 2). Of the 28 included studies, 8 reported random sequence generation and allocation concealment, while 14 reported blinding participants and personnel, 9 reported blinding outcome assessors, and 10 were considered to have a low risk for incomplete outcome data reporting. Adverse events were reported in 13 studies. The risk of bias due to sources of funding was low in 11 studies.
| Table 1 Characteristics of included studies |
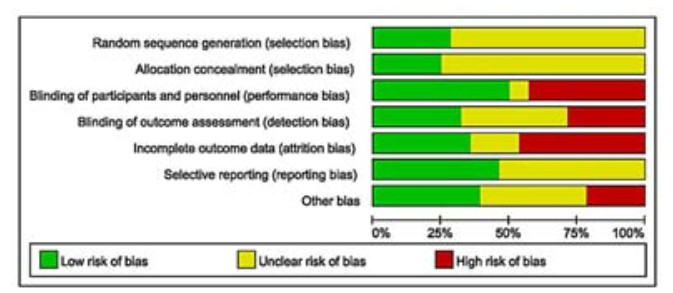
|
Figure 2 Summary of risk of bias assessment. |
Three studies of 13 788 participants evaluated the effects of statins on ESRD events (Fig. 3). These studies showed no substantial heterogeneity (I2=0, P=0.78) and reported little or no effect of statins in preventing progression of CKD to ESRD (RR 0.98, 95% CI: 0.91-1.05). We did not perform subgroup analyses because of the small number of studies included.
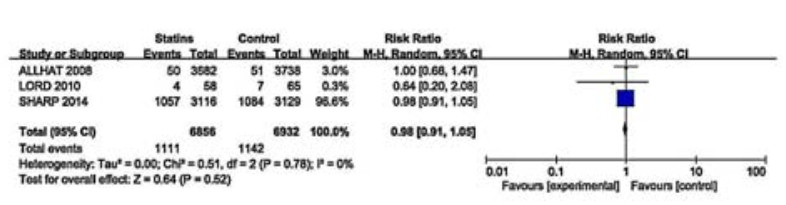
|
Figure 3 Effects of statins compared with control on progression to ESRD in adults with CKD not requiring dialysis therapy. |
Two studies of 9512 participants reported that statin therapy showed no beneficial effect on doubling of serum creatinine level (RR 1.43, 95% CI: 0.26=7.79; Fig. 4). These two studies showed a moderate heterogeneity (I2=46%, P=0.17) and we did not perform subgroup analyses for the small sample size.
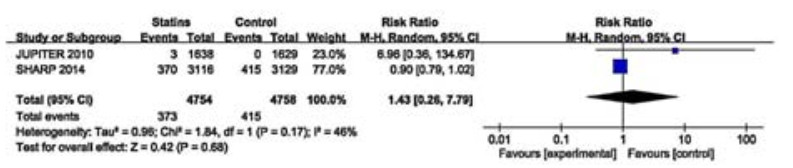
|
Figure 4 Effects of statins compared with control on doubling of serum creatinine level in adults with CKD not requiring dialysis therapy. |
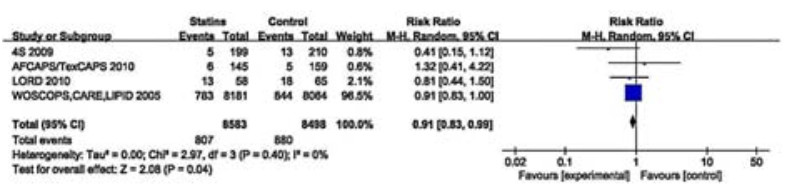
The results of 4 studies involving 17 081 participants showed a probable beneficial effect of statin therapy on eGFR reduction by over 25% (RR 0.91, 95% CI: 0.83-0.99; Fig. 5). These studies showed no significant heterogeneity (I2=0, P=0.40) and we did not perform subgroup analyses of these studies.
|
Figure 5 Effects of statins compared with control on an eGFR reduction by over 25% in adults with CKD not requiring dialysis therapy. |
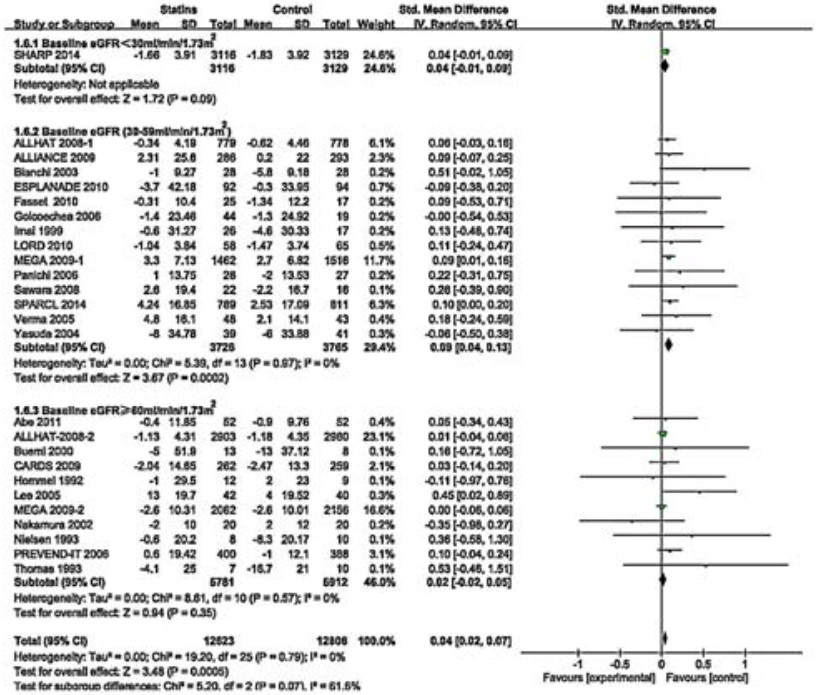
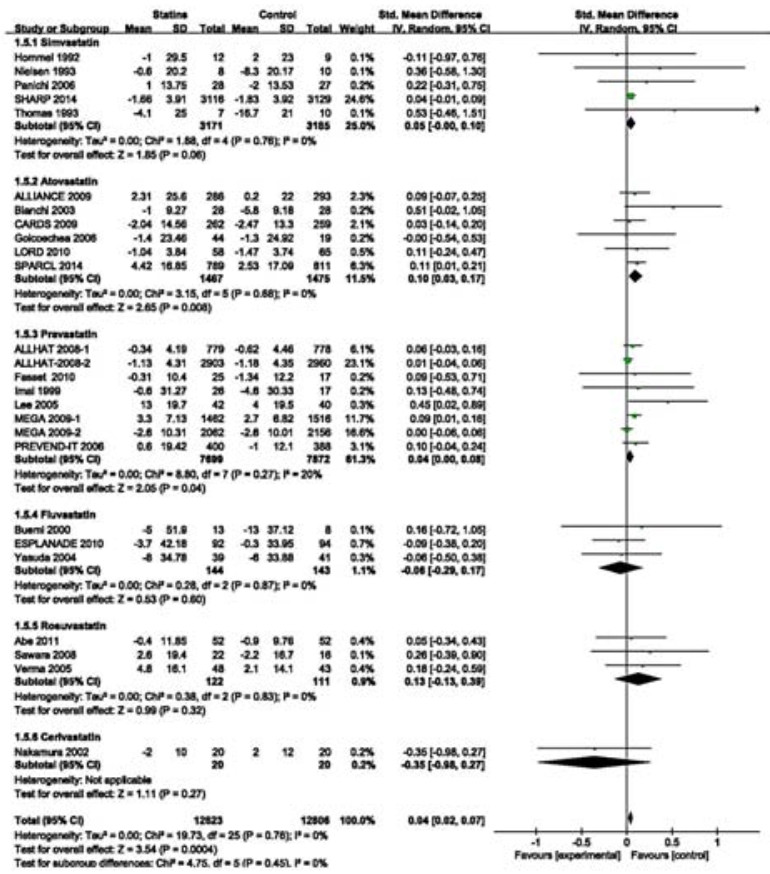
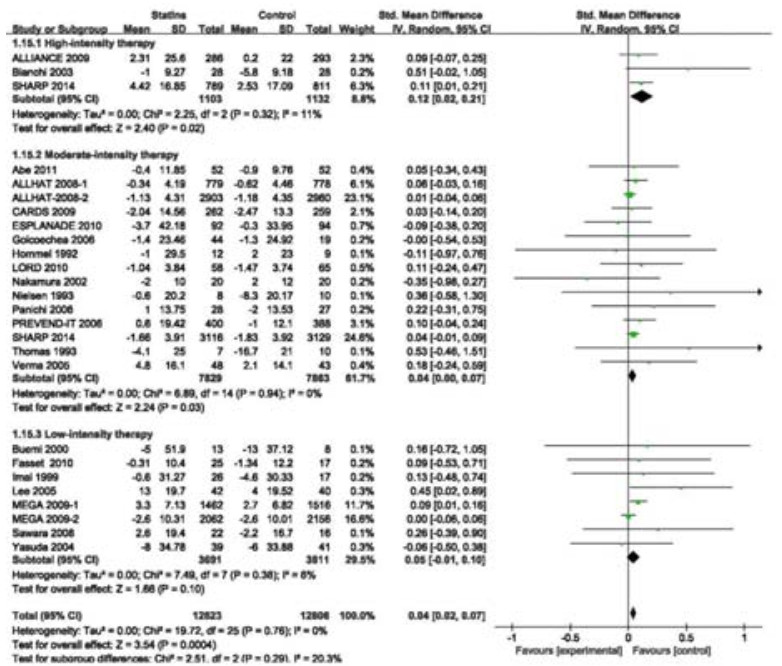
Twenty-four RCTs (including a subgroup in ALLHAT 2008 and one in MEGA 2009) involving 25 429 participants estimated the effects of statins on changes of eGFR. Overall, the SMD for the effects on eGFR changes was statistically significant (SMD 0.04, 95% CI: 0.02-0.07; Fig. 6). These RCTs had no heterogeneity (I2=0%, P=0.79). To explore the effects of baseline eGFR and statin types on eGFR changes, we conducted a subgroup analysis according to baseline eGFR and statin types. The result showed that the benefit of statin therapy was statistically significant in moderate CKD subgroup of 7491 participants (SMD 0.09, 95% CI: 0.04=0.13; I2=0%, P=0.97; Fig. 6). However, statin therapy had no significant effect on eGFR changes in mild (11 693 participants; SMD 0.02, 95% CI: -0.02-0.05; I2=0%, P=0.57) and severe[only one study (SHARP 2014) of 6 245 participants; SMD 0.04, 95% CI:-0.01-0.09] CKD subgroups. Among these statins, only atorvastatin had a beneficial effect on eGFR changes (SMD 0.10, 95% CI 0.03-0.17) (Fig. 7). However, the effects of other statins were not significant [simvastatin: SMD 0.05, 95% CI:-0.00-0.10; pravastatin: SMD 0.04, 95% CI: 0.00-0.08; fluvastatin: SMD-0.06, 95% CI:-0.29-0.17; rosuvastatin: SMD 0.13, 95% CI: -0.13 to 0.39; cerivastatin (only one study); SMD -0.35, 95% CI: -0.98-0.27]. The benefit of statin therapy on eGFR changes was statistically significant in studies of high-intensity statin as compared with controls (SMD 0.12, 95% CI: 0.02-0.21; Fig. 8). No statistically significant difference of eGFR changes was found in patients receiving moderateintensity (SMD 0.04, 95% CI: 0.00-0.07) or low-intensity (SMD 0.05, 95% CI: -0.01-0.10) therapy. In sensitivity analysis, we found that the results were robust when only those studies reporting blinding techniques were included. Statin therapy still had a favorable effect when trials with follow-up periods < 1 year were excluded (SMD 0.04, 95% CI: 0.02-0.07).
|
Figure 6 Changes in standardized mean differences (SMD) of eGFR in patients with statin therapy compared with controls. |
|
Figure 7 Changes in standardized mean differences (SMD) of eGFR in patients receiving different statins compared with controls. |
|
Figure 8 Changes in the standardized mean differences (SMD) of eGFR in patients receiving statins therapy of different intensities compared with controls. |
There was no asymmetry of the funnel plot regarding eGFR changes, suggesting the absence of publication bias in the included studies (data not shown). Although we planned to examine publication bias of other outcomes using funnel plots, the small number of included studies prohibited this[22].
DISCUSSIONThe findings of this meta-analysis of the 28 studies suggest that statins appeared to have little or no effect on doubling of serum creatinine level and in preventing progression of CKD to ESRD, but can be beneficial to improve GFR or reduce the decline of GFR.
Hyperlipidemia may play an important role in the progression of kidney disease[4, 6, 8] by exacerbating loss of kidney function and damaging the glomerular basement membrane [52]. Statins are known to have pleiotropic effects[9, 15] and their renoprotective effects are probably mediated by improving endothelial dysfunction [53], increasing renal perfusion[14], modulating inflammatory response to dyslipidemia [53], and attenuating cell apoptosis[9].
Previous studies that evaluated the effects of statins on progression of CKD yielded inconsistent results. Some studies reported benefits of statins on slowing down GFR reduction[31, 42, 51], while some others did not show any benefits to the renal outcomes[20, 45, 47]. The first meta-analysis of 13 studies by Fried et al [54] found a modest improvement of kidney function in patients receiving statin therapy, but this finding did not seem convincing given the small number of studies and participants included, short duration of observation and poor study quality. In another meta-analysis of 27 randomized trials, Sandhu et al [16] found that statin therapy modestly slowed down the rate of kidney function loss by 1.22 mL/min per year, but apart from the substantial heterogeneity of the studies included, they failed to provide the clinically relevant outcome of interest: progression of CKD to ESRD. The findings of a Cochrane systematic review have indicated that statins have uncertain effects on progression of kidney disease [19]. In this meta-analysis, we studied the clinically relevant outcomes and found that statin therapy had little or no effect on doubling of serum creatinine levels or progression of CKD to ESRD. We also studied the effects of statins on eGFR reduction by over 25% and found that statin therapy may be beneficial to improve GFR in CKD patients.
Based on the overall pooled data from 24 studies of 25 429 participants, we found beneficial effects of statin therapy on eGFR changes in CKD patients. A subgroup analysis of the Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese (MEGA) study suggested that the effect of statin therapy is subjected to influences by baseline eGFR and pravastatin may prove beneficial on renal function in patients with moderate CKD[44]. In our meta-analysis, we took baseline eGFR into consideration and found that statin treatment did not significantly reduce kidney function loss in patients with mild or severe CKD, but showed a significant beneficial effect in the much larger subgroup of moderate CKD, suggesting the potential benefit of statins for patients with moderate CKD. To evaluate the drug-specific effects on renal function, we conducted a subgroup analysis by statin types and found that only atorvastatin produced a beneficial effect on eGFR changes, which was consistent with the finding in a previous meta-analysis[18]. The effects of statin therapy on kidney function are associated with statin doses. According to Sanguankeo et al[55], a high-intensity statin therapy was associated with improved kidney function, which was consistent with our findings in subgroup analysis that high-intensity statin therapy produced a significant beneficial effect on eGFR changes as compared with moderate-and low-intensity statin therapies. Compared with the study by Sanguankeo et al [55], we included more RCTs with large sample sizes without substantial heterogeneity among the studies. Our results demonstrate that reduced LDL-C levels are significantly associated with eGFR changes and suggest a probable dose-response effect of statins in CKD patients.
We noted that the outcomes of ESRD events and doubling of serum creatinine level were inconsistent with the outcomes of kidney functions. This inconsistency might arise from the difference in the studies included in the analysis, as we analyzed 24 studies for the effects of statins on eGFR changes, but only 3 studies and 2 studies were available that examined the effects of statins on progression of CKD to ESRD and on doubling of serum creatinine level, respectively. The difference in clinical characteristics of the study participants across the analyses also contribute to the inconsistency in the outcomes. For instance, the mean baseline eGFR was 26.6 mL/min/ 1.73 m2 in the SHARP study[20]as compared to 30.5 mL/min/ 1.73 m2 in the LORD study[46]. In the SHARP study, the high proportion (63%) of non-dialysis patients with stage 4 or 5 CKD at randomization possibly diminished the beneficial effects of statin therapy on progression of CKD to ESRD. The differences across the studies in statin types used, the intensity of statin therapy, and the duration of patient follow-up are all factors that may influence the overall effects of statins on the progression of CKD. The LORD study, for instance, found that the decline in GFR was lessened during the first 2 years and at the end of the follow-up period, and no significant differences were found between the statin and placebo groups[46].
There were some limitations in our analysis. First, we included post-hoc analyses of CKD subgroups from RCTs not specifically designed to assess patients with CKD, which may have introduced bias; Second, few of the included studies directly addressed the effects of statins on renal outcomes in CKD patients; Third, the measurement of kidney function differed among some RCTs; Fourth, although there was no substantial heterogeneity in this study, we should not ignore the differences among the RCTs, especially the clinical heterogeneity; Lastly, in some studies, patients also received concomitant therapy, which may introduce bias to our results.
ConclusionAlthough statins may not prevent the progression of CKD to ESRD and the doubling of the serum creatinine level, they appear to improve GFR or reduce the decline in GFR in CKD patients. These effects of statins are probably associated with the patients' baseline eGFR level, statin types used, and the intensity of statin therapy received.
| [1] |
Maneesh S, Navdeep T, Melania P, et al. Risk of end-stage renal disease and death after cardiovascular events in chronic kidney disease[J].
Circulation,2014, 130 (6) : 458-65.
DOI: 10.1161/CIRCULATIONAHA.113.007106. ( 0) 0)
|
| [2] |
Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk:epidemiology, mechanisms, and prevention[J].
Lancet,2013, 382 (9889) : 339-52.
DOI: 10.1016/S0140-6736(13)60595-4. ( 0) 0)
|
| [3] |
Braun L, Sood V, Hogue S, et al. High burden and unmet patientneeds in chronic kidney disease[J].
Int J Nephrol Renovasc Dis,2012, 5 (5) : 151-63.
( 0) 0)
|
| [4] |
Iseki K. Epidemiology of dyslipidemia in chronic kidney disease[J].
Clin Exp Nephrol,2014, 18 (2) : 185-8.
DOI: 10.1007/s10157-013-0891-8. ( 0) 0)
|
| [5] |
Upadhyay A. Statins in chronic kidney disease:what do metaanalyses tell us[J].
Clin Exp Nephrol,2014, 18 (2) : 278-81.
DOI: 10.1007/s10157-013-0889-2. ( 0) 0)
|
| [6] |
Chen S, Hung C, Kuo M, et al. Association of dyslipidemia with renal outcomes in chronic kidney disease[J].
PLoS One,2013, 8 (2) : e55643.
DOI: 10.1371/journal.pone.0055643. ( 0) 0)
|
| [7] |
Gluba A, Rysz J, Banach M. Statins in patients with chronic kidney disease:why, who and when[J].
Expert Opin Pharmacother,2010, 11 (16) : 2665-74.
DOI: 10.1517/14656566.2010.512419. ( 0) 0)
|
| [8] |
Liu ZS, Liu DW. Dyslipidemia promotes the progression of chronic kidney disease[J].
Chin J Med (Engl),2013, 126 (7) : 1203-6.
( 0) 0)
|
| [9] |
Nam HK, Lee SJ, Kim MH, et al. Rosuvastatin attenuates inflammation, apoptosis and fibrosis in a rat model of cyclosporineinduced nephropathy[J].
Am J Nephrol,2013, 37 (1) : 7-15.
DOI: 10.1159/000345990. ( 0) 0)
|
| [10] |
Yen C, Sun C, Leu S, et al. Continuing exposure to low-dose nonylphenol aggravates adenine-induced chronic renal dysfunction and role of rosuvastatin therapy[J].
J Transl Med,2012, 10 (1) : 147.
DOI: 10.1186/1479-5876-10-147. ( 0) 0)
|
| [11] |
Hamasaki Y, Doi K, Okamoto K, et al. 3-Hydroxy-3-me-thylglutarylcoenzyme A reductase inhibitor simvastatin ameliorates renal fibrosis through HOXA13-USAG-1 pathway[J].
Lab Invest,2012, 92 (8) : 1161-70.
DOI: 10.1038/labinvest.2012.71. ( 0) 0)
|
| [12] |
Sonmez A, Yilmaz MI, Korkmaz A, et al. Hyperbaric oxygen treatment augments the efficacy of cilazapril and simvastatin regimens in an experimental nephrotic syndrome model[J].
Clin Exp Nephrol,2008, 12 (2) : 110-8.
DOI: 10.1007/s10157-007-0017-2. ( 0) 0)
|
| [13] |
de Barros ÉP, Garcia-Pinto AB, Machado PY, et al. Rosuvastatin beneficially alters the glomerular structure of kidneys from spontaneously hypertensive rats (SHRs)[J].
J Mol Histol,2011, 42 (4) : 323-31.
DOI: 10.1007/s10735-011-9336-4. ( 0) 0)
|
| [14] |
Kamdar C, Chou S, Mooppan UMM, et al. Atorvastatin protects renal function in the rat with acute unilateral ureteral obstruction[J].
Urology,2010, 75 (4) : 853-7.
DOI: 10.1016/j.urology.2009.05.030. ( 0) 0)
|
| [15] |
Giunti S, Calkin AC, Forbes JM, et al. The pleiotropic actions of rosuvastatin confer renal benefits in the diabetic Apo-E knockout mouse[J].
Am J Physiol Renal Physiol,2010, 299 (3) : F528-35.
DOI: 10.1152/ajprenal.00127.2010. ( 0) 0)
|
| [16] |
Sabrina S, Natasha W, Fried LF, et al. Statins for improving renal outcomes:a meta-analysis[J].
J Am Soc Nephrol,2006, 17 (7) : 2006-16.
DOI: 10.1681/ASN.2006010012. ( 0) 0)
|
| [17] |
Nikolic D, Banach M, Nikfar S, et al. A meta-analysis of the role of statins on renal outcomes in patients with chronic kidney disease[J].
Is the duration of therapy important[J]? Int J Cardiol,2013, 168 (6) : 5437-47.
( 0) 0)
|
| [18] |
Takagi H, Umemoto T. A meta-analysis of randomized trials for effects of atorvastatin on renal function in chronic kidney disease[J].
Int J Cardiol,2011, 152 (2) : 242-4.
DOI: 10.1016/j.ijcard.2011.06.107. ( 0) 0)
|
| [19] |
Navaneethan SD, Pansini F, Perkovic V, et al. HMG CoA reductase inhibitors (statins) for people with chronic kidney disease not requiring dialysis[J].
Cochrane Database Syst Rev,2009, 5 (2) : D7784.
( 0) 0)
|
| [20] |
Richard H, David L, Jonathan E, et al. Effects of lowering LDL cholesterol on progression of kidney disease[J].
J Am Soc Nephrol,2014, 25 (8) : 1825-33.
DOI: 10.1681/ASN.2013090965. ( 0) 0)
|
| [21] |
Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease:evaluation, classification, and stratification[J].
Ann Intern Med,2003, 139 (2) : 137-47.
DOI: 10.7326/0003-4819-139-2-200307150-00013. ( 0) 0)
|
| [22] |
Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0[updated March 2011]. The Cochrane Collaboration, 2011.[Available at www.cochranehandbook.org]
( 0) 0)
|
| [23] |
Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses[J].
BMJ,2003, 327 (7414) : 557-60.
DOI: 10.1136/bmj.327.7414.557. ( 0) 0)
|
| [24] |
Ray KK, Kastelein JJP, S Matthijs B, et al. The ACC/AHA 2013 guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults:the good the bad and the uncertain:a comparison with ESC/EAS guidelines for the management of dyslipidaemias 2011[J].
Eur Heart J,2014, 35 (15) : 960-8.
DOI: 10.1093/eurheartj/ehu107. ( 0) 0)
|
| [25] |
Hommel E, Andersen P, Gall MA, et al. Plasma lipoproteins and renal function during simvastatin treatment in diabetic nephropathy[J].
Diabetologia,1992, 35 (5) : 447-51.
DOI: 10.1007/BF02342442. ( 0) 0)
|
| [26] |
Nielsen S, Schmitz O, Møller N, et al. Renal function and insulin sensitivity during simvastatin treatment in type 2(non-insulin-dependent) diabetic patients with microalbuminuria[J].
Diabetologia,1993, 36 (10) : 1079-86.
DOI: 10.1007/BF02374502. ( 0) 0)
|
| [27] |
Thomas ME, Harris KP, Ramaswamy C, et al. Simvastatin therapy for hypercholesterolemic patients with nephrotic syndrome or significant proteinuria[J].
Kidney Int,1993, 44 (5) : 1124-9.
DOI: 10.1038/ki.1993.358. ( 0) 0)
|
| [28] |
Imai Y, Suzuki H, Saito T, et al. The effect of pravastatin on renal function and lipid metabolism in patients with renal dysfunction with hypertension and hyperlipidemia[J].
Clin Exp Hypertens,1999, 21 (8) : 1345-55.
DOI: 10.3109/10641969909070853. ( 0) 0)
|
| [29] |
Buemi M, Allegra A, Corica F, et al. Effect of fluvastatin on proteinuria in patients with immunoglobulin A nephropathy[J].
Clin Pharmacol Ther,2000, 67 (4) : 427-31.
DOI: 10.1067/mcp.2000.105330. ( 0) 0)
|
| [30] |
Nakamura T, Ushiyama C, Hirokawa K, et al. Effect of cerivastatin on proteinuria and urinary podocytes in patients with chronic glomerulonephritis[J].
Nephrol Dial Transplant,2002, 17 (5) : 798-802.
DOI: 10.1093/ndt/17.5.798. ( 0) 0)
|
| [31] |
Bianchi S, Bigazzi R, Caiazza A, et al. A controlled, prospective study of the effects of atorvastatin on proteinuria and progression of kidney disease[J].
Am J Kidney Dis,2003, 41 (3) : 565-70.
DOI: 10.1053/ajkd.2003.50140. ( 0) 0)
|
| [32] |
Yasuda G, Kuji T, Hasegawa K, et al. Safety and efficacy of fluvastatin in hyperlipidemic patients with chronic renal disease[J].
Ren Fail,2004, 26 (4) : 411-8.
DOI: 10.1081/JDI-120039826. ( 0) 0)
|
| [33] |
Lee T, Lin M, Tsai C, et al. Add-on and withdrawal effect of pravastatin on proteinuria in hypertensive patients treated with AT1 receptor blockers[J].
Kidney Int,2005, 68 (2) : 779-87.
DOI: 10.1111/j.1523-1755.2005.00457.x. ( 0) 0)
|
| [34] |
Tonelli M, Isles C, Craven T. Effect of pravastatin on rate of kidney function loss in people with or at risk for coronary disease[J].
Circulation,2005, 112 (2) : 171-8.
DOI: 10.1161/CIRCULATIONAHA.104.517565. ( 0) 0)
|
| [35] |
Verma A, Ranganna KM, Reddy RS, et al. Effect of rosuvastatin on C-reactive protein and renal function in patients with chronic kidney disease[J].
Am J Cardiol,2005, 96 (9) : 1290-2.
DOI: 10.1016/j.amjcard.2005.06.074. ( 0) 0)
|
| [36] |
Atthobari J, Brantsma AH, Gansevoort RT, et al. The effect of statins on urinary albumin excretion and glomerular filtration rate:results from both a randomized clinical trial and an observational cohort study[J].
Nephrol Dial Transplant,2006, 21 (11) : 3106-14.
DOI: 10.1093/ndt/gfl244. ( 0) 0)
|
| [37] |
Marian G, Soledad García DV, Vicente L, et al. Effects of atorvastatin on inflammatory and fibrinolytic parameters in patients with chronic kidney disease[J].
J Am Soc Nephrol,2006, 17 (12Suppl3) : S231-5.
( 0) 0)
|
| [38] |
Panichi V, Paoletti S, Mantuano E, et al. In vivo and in vitro effects of simvastatin on inflammatory markers in pre-dialysis patients[J].
Nephrol Dial Transplant,2006, 21 (2) : 337-44.
DOI: 10.1093/ndt/gfi224. ( 0) 0)
|
| [39] |
Rahman M, Baimbridge C, Davis BR, et al. Progression of kidney disease in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin versus usual care:a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)[J].
Am J Kidney Dis,2008, 52 (3) : 412-24.
DOI: 10.1053/j.ajkd.2008.05.027. ( 0) 0)
|
| [40] |
Sawara Y, Takei T, Uchida K, et al. Effects of lipid-lowering therapy with rosuvastatin on atherosclerotic burden in patients with chronic kidney disease[J].
Intern Med,2008, 47 (17) : 1505-10.
DOI: 10.2169/internalmedicine.47.1159. ( 0) 0)
|
| [41] |
Colhoun HM, Betteridge DJ, Durrington PN, et al. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes:an analysis from the Collaborative Atorvastatin Diabetes Study (CARDS)[J].
Am J Kidney Dis,2009, 54 (5) : 810-9.
DOI: 10.1053/j.ajkd.2009.03.022. ( 0) 0)
|
| [42] |
Huskey J, Lindenfeld J, Cook T, et al. Effect of simvastatin on kidney function loss in patients with coronary heart disease[J].
Atherosclerosis,2009, 205 (1) : 202-6.
DOI: 10.1016/j.atherosclerosis.2008.11.010. ( 0) 0)
|
| [43] |
Koren MJ, Davidson MH, Wilson DJ, et al. Focused atorvastatin therapy in managed-care patients with coronary heart disease and CKD[J].
Am J Kidney Dis,2009, 53 (5) : 741-50.
DOI: 10.1053/j.ajkd.2008.11.025. ( 0) 0)
|
| [44] |
Nakamura H, Mizuno K, Ohashi Y, et al. Pravastatin and cardiovascular risk in moderate chronic kidney disease[J].
Atherosclerosis,2009, 206 (2) : 512-7.
DOI: 10.1016/j.atherosclerosis.2009.03.031. ( 0) 0)
|
| [45] |
Fassett RG, Coombes JS, Packham D, et al. Effect of pravastatin on kidney function and urinary protein excretion in autosomal dominant polycystic kidney disease[J].
Scand J Urol Nephrol,2009, 44 (1) : 56-61.
( 0) 0)
|
| [46] |
Fassett RG, Robertson IK, Ball MJ, et al. Effect of atorvastatin on kidney function in chronic kidney disease:a randomised doubleblind placebo-controlled trial[J].
Atherosclerosis,2010, 213 (1) : 218-24.
DOI: 10.1016/j.atherosclerosis.2010.07.053. ( 0) 0)
|
| [47] |
Kendrick J, Shlipak MG, Targher G, et al. Effect of lovastatin on primary prevention of cardiovascular events in mild ckd and kidney function loss:a post hoc analysis of the Air Force/Texas Coronary Atherosclerosis Prevention Study[J].
Am J Kidney Dis,2010, 55 (1) : 42-9.
DOI: 10.1053/j.ajkd.2009.09.020. ( 0) 0)
|
| [48] |
Ridker PM, Macfadyen J, Cressman M, et al. Efficacy of rosuvastatin among men and women with moderate chronic kidney disease and elevated high-sensitivity C-Reactive protein[J].
J Am Coll Cardiol,2010, 55 (12) : 1266-73.
DOI: 10.1016/j.jacc.2010.01.020. ( 0) 0)
|
| [49] |
Ruggenenti P, Perna A, Tonelli M, et al. Effects of add-on fluvastatin therapy in patients with chronic proteinuric nephropathy on dual renin-angiotensin system blockade:the ESPLANADE trial[J].
Clin J Am Soc Nephrol,2010, 5 (11) : 1928-38.
DOI: 10.2215/CJN.03380410. ( 0) 0)
|
| [50] |
Abe M, Maruyama N, Okada K, et al. Effects of lipid-lowering therapy with rosuvastatin on kidney function and oxidative stress in patients with diabetic nephropathy[J].
J Atheroscler Thromb,2011, 18 (11) : 1018-28.
( 0) 0)
|
| [51] |
Pierre A, Alfred C, Campese VM, et al. Effect of high-dose atorvastatin on renal function in subjects with stroke or transient ischemic attack in the SPARCL trial[J].
Stroke,2014, 45 (10) : 2974-82.
DOI: 10.1161/STROKEAHA.114.005832. ( 0) 0)
|
| [52] |
Moorhead JF. Lipids and progressive kidney disease[J].
Kidney Int Suppl,1991, 31 (4) : S35-40.
( 0) 0)
|
| [53] |
Fiore MC, Jimenez PM, Cremonezzi D, et al. Statins reverse renal inflammation and endothelial dysfunction induced by chronic high salt intake[J].
Am J Physiol Renal Physiol,2011, 301 (2) : F263-70.
DOI: 10.1152/ajprenal.00109.2010. ( 0) 0)
|
| [54] |
Fried LF, Orchard TJ, Kasiske BL. Effect of lipid reduction on the progression of renal disease:a meta-analysis[J].
Kidney Int,2001, 59 (1) : 260-9.
DOI: 10.1046/j.1523-1755.2001.00487.x. ( 0) 0)
|
| [55] |
Sanguankeo A, Upala S, Cheungpasitporn W, et al. Effects of statins on renal outcome in chronic kidney disease patients:a systematic review and meta-analysis[J].
PLoS One,2015, 10 (7) : e132970.
( 0) 0)
|
 2016, Vol. 36
2016, Vol. 36
