2. 南方医科大学 第一临床医学院,广 东 广州 510515
2. First School of Clinical Medicine, Southern Medical University, Guangzhou 510515, China
产单核细胞李斯特菌(Listeria monocytogenes, LM)简称单增李斯特菌,是李斯特菌属(listeria)中唯一对人类致病的兼性胞内寄生菌,能引起全身多器官的感染,病死率极高,免疫功能较弱人群更容易感染并致病[1-2]。目前抗生素广泛而有效地应用于各种感染性疾病的治疗,针对李斯特菌感染的治疗药物多为青霉素类、头孢菌素类、磺胺甲噁唑等胞外抗生素[3-5],这些抗生素无法进入细胞内杀菌并且存在于细胞内的细菌可以逃避机体的免疫防御及清除等问题使得人体感染LM后导致较高的发病率和病死率[6]。面对LM感染缺乏有效治疗药物的严峻挑战,本文首次提出通过c-Met受体阻断剂XL-184抑制LM对宿主细胞的侵袭以预防和治疗LM感染。
研究发现LM毒力因子内化素B(InlB)可模拟肝生长因子与其配体肝生长因子受体或分散因子受体(c-Met)结合,从而导致细菌入侵无吞噬能力的上皮细胞并引起信号通路的激活[7-8]。C-Met受体抑制剂可特异性阻断LM进入细胞,其中卡博替尼(XL-184)是一种新型c-Met受体阻断剂,2012年获FDA批准上市用于治疗晚期或转移性甲状腺髓样癌[9]。XL-184作为抗癌药物可能具有较大的药物副作用,但面对LM严重感染素手无策的困境,仍不失为一种较好的策略,因此XL-184可作为一种潜在的预防和治疗LM感染的新型抗菌药物进行研发。LM侵袭Caco-2后可降低细胞之间紧密连接的牢固性并增大细胞膜的通透性[10],进一步引起细胞损伤及死亡。不同浓度及孵育时间的XL-184作用于Caco-2细胞可能会在不同程度上降低LM介导的损伤作用。本文利用Caco-2细胞模拟肠屏障,观察XL-184药物剂量和药物作用时间对于细菌侵袭、细胞通透性及细胞存活率等的影响,初步探索XL-184在阻止LM侵袭Caco-2细胞的过程中所起的作用。
1 材料和方法 1.1 菌株及细胞LM菌株ATCC株13932(血清型4b)由美国南加州大学洛杉矶儿童医院黄胜和教授提供并由本室保存,Caco-2细胞购于上海细胞库。
1.2 主要试剂卡博替尼(XL-184)购于美国Selleck Chemicals,Transwell小室(3 μm)购自美国Corning,乳酸脱氢酶(LDH)试剂盒为苏州科铭生物技术有限公司产品。胎牛血清、BHI培养基、辣根过氧化物酶(HRP)分别为德国PAN、美国BD公司、北京鼎国昌盛生物技术有限责任公司产品。
1.3 XL-184对LM生长曲线的影响BHI培养基中加入0~10 μmol/L的XL-184,并将LM培养于其中,37 ℃摇床孵育,于0~16 h测量细菌D值。
1.4 LM侵袭率分析(1)侵袭实验方法参考文献[11],设计两组实验验证XL-184抑制LM侵袭Caco-2细胞的能力:单独用药组和联合用药组。单独用药组中,培养好的Caco-2(约105/孔)加入0~10 μmol/L XL-184并孵育1~48 h。联合用药组设两组,第1组为各浓度XL-184分别与50 μg/mL氨苄西林(Ampicillin, Amp)共孵育细胞24 h;第2组为各浓度Amp分别与0.1 μmol/L XL-184共孵育细胞24 h。(2)药物孵育完毕加入LM(细菌/细胞数比值为100,MOI=100),37 ℃孵育1.5 h,对照组另加入DH5a、BL21(DE3)以比较致病菌和非致病菌的侵袭率。加入庆大霉素,37 ℃共孵育1 h。加入100 μL 5% TritonX-100裂解细胞8 min,蒸馏水终止裂解。(3)将细胞裂解液稀释并涂板,37 ℃培养24 h后计数,计算相对侵袭率,重复3次取均值。
1.5 单层细胞通透性分析(1)将Caco-2细胞接种于Transwell小室上层,生长于聚碳酸酯膜的Caco-2细胞可将transwell分为上下室,上、下室可分别视为模拟肠道和血管内环境[12];(2)上室加入0、25、50、100 nmol/L的XL-184,孵育24 h,加入细菌(MOI=100)孵育1.5 h,加入HRP 3 μg [13]37 ℃孵育;(3)分别于0、2、4、6 h从下层取30 μL培养基,上机检测下室HRP的浓度,计数下室培养基中细菌CFU,并测量TEER值[14]。
1.6 LDH释放率分析实验方法参考文献[15]。含0~10 μmol/L XL-184的培养基孵育细胞1、24 h,加入LM(MOI=100)37 ℃孵育1.5 h,取10 μL上清于96孔板中,测量上清LDH浓度。然后加入10% Triton-X100裂解细胞,取10 μl细胞裂解液于96孔板,用于测量细胞内LDH含量。LDH含量测定依据检测试剂盒说明书,于450 nm下检测吸光度。
1.7 细胞活性分析XL-184(2.5、0.5、0 μmol/L)孵育细胞24 h,加入LM(MOI=100),37 ℃孵育1.5 h,PBS清洗3遍,暗室内加入稀释的DAPI染液15 μL [16]和稀释的PI染液15 μL [17],30 min后上机检测。
1.8 统计学分析使用SPSS 13.0统计软件进行统计学分析,计量资料以均数±标准差表示,计数资料以百分比表示,统计学方法采用完全随机设计资料的方差分析及两因素方差分析,P < 0.05为差异有统计学意义,各实验至少重复3遍。
2 结果 2.1 XL-184对LM生长曲线的影响LM生长曲线(图 1)表明:含不同浓度XL-184的培养基中LM的生长情况无差异,因此XL-184对LM体外生长没有抑制作用,本实验观察到的药物所致的细胞效应由XL-184作用于Caco-2细胞引起。
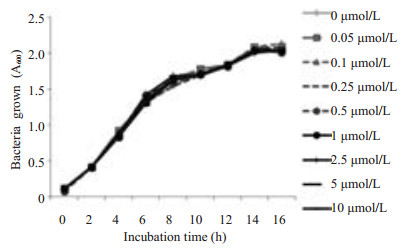
|
图 1 不同XL-184浓度下LM生长曲线 Figure 1 Growth curves of LM in the presence of different concentrations of XL-184. Bacteria growth in BHI broth was monitored by measuring the absorbance of liquid cultures at 600 nm (D600). Similar results were obtained from bacterial cultures grown in MEM containing 10% FBS |
在培养好的Caco-2细胞中分别加入LM、DH5a、BL21(DE3)(MOI=100),细菌粘附1.5 h,加入庆大霉素孵育,裂解细胞并涂板计数。结果显示LM侵袭率约为0.168%,DH5α的侵袭率为0.019%,BL21(DE3)的侵袭率为0.023%,LM侵袭率高于对照组DH5α、BL21(DE3),P=0.000。
2.3 XL-184对于LM侵袭Caco-2细胞的影响将含XL-184的培养基孵育细胞1~48 h,进行侵袭实验,随着XL-184浓度升高,LM侵袭Caco-2的侵袭率降低(图 2)。药物组侵袭率均低于对照组(P=0.000)。相同剂量药物作用下,XL-184孵育Caco-2细胞1,2,6,12 h即可降低LM的侵袭率,XL-184孵育Caco-2细胞24 h侵袭率明显降低,XL-184孵育Caco-2细胞48 h和24 h相比侵袭率降低不明显。
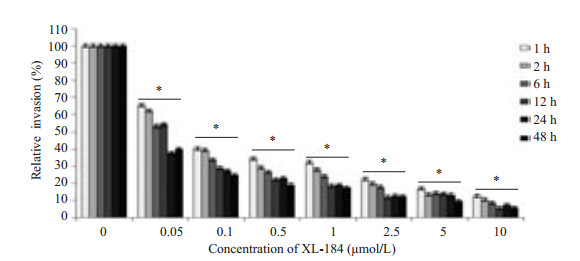
|
图 2 XL-184剂量对于LM侵袭Caco-2的影响 Figure 2 Dose-dependent inhibitory effect of XL-184 on LM invasion in Caco-2 cells. Caco-2 cells were incubated with various concentrations of XL-184 for 1 to 48 h before bacterial infection. Results of invasion assays are expressed as the percentage of LM invasion relative to that in the control cells (without XL-184 incubation). Error bars indicate standard deviations. *P=0.000 vs control group. |
联合用药第1组(图 3A): XL-184浓度为0~5 μmol/L时,实验组LM对细胞的侵袭率低于对照组(P0~1 < 0.01,P5 < 0.05),随XL-184剂量增大,LM对Caco-2的侵袭率有下降趋势。联合用药第2组(图 3B):各浓度氨苄西林作用下,实验组LM对细胞的侵袭率均低于对照组(P < 0.01),随氨苄西林剂量增大,LM对Caco-2的侵袭率有下降趋势。
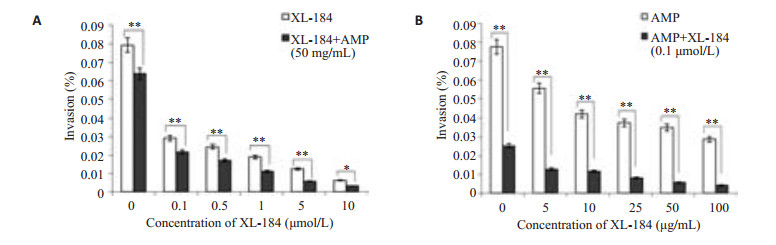
|
图 3 XL-184与Amp联合用药对LM侵袭Caco-2细胞的影响 Figure 3 XL-184 potentiates intracellular killing of LM in Caco-2 cells co-incubated with AMP. Caco-2 cells were incubated with both XL-184 and AMP at the specified concentrations. The intracellular killing activity was significantly higher with a combined treatment with AMP and XL-184 than with either of them alone. Error bars indicate standard deviations. *P < 0.05, **P < 0.01. |
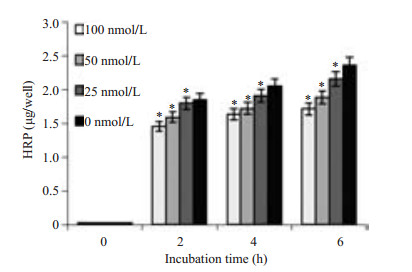
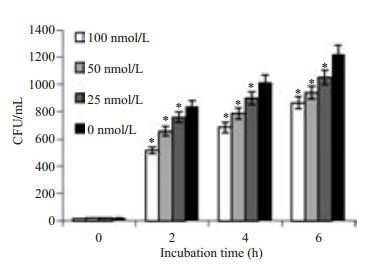
Caco-2细胞是人结肠腺癌细胞,细胞之间可以形成紧密连接,以抵挡肠道中有害因素入侵人体,LM可与Caco-2表面c-Met结合引起单层细胞通透性的变化,可通过检测TEER值(图 4)、下室HRP浓度(图 5)及细菌CFU的变化(图 6)预测单层细胞通透性的改变情况。通过实验我们观察到:随XL-184浓度升高TEER值下降减缓(P < 0.05),下室培养基中HRP浓度降低,并且LM数量变少(P=0.000)。同时随LM侵袭Caco-2细胞时间的延长,TEER值降低幅度增大,下室HRP浓度升高,下室细菌数量增多。
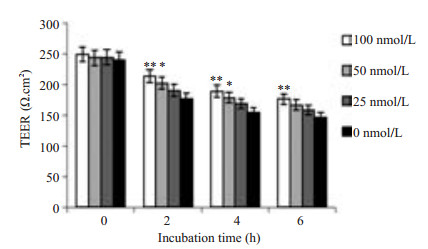
|
图 4 XL-184剂量对Caco-2细胞TEER值的影响 Figure 4 Dose-dependent effect of XL-184 on TEER value in Caco-2 cells. Caco-2 cells were incubated with different doses of XL-184 for 24 h and equivalent amount of LM [corresponding to 0-100 multiplicity of infection (MOI)] for 1.5 h. Cells without XL-184 treatment served as the control. Error bars indicate standard deviations. *P < 0.05, **P < 0.01 vs control group. |
|
图 5 XL-184剂量对下室HRP浓度的影响 Figure 5 Dose-dependent effect of XL-184 on HRP concentration in the lower chamber. Caco-2 cells were treated with different doses of XL-184 for 24 h and then with equivalent amount of LM for 1.5 h. Cells without XL-184 treatment served as the control. Error bars indicate standard deviations. *P=0.000 vs control group. |
|
图 6 XL-184剂量对下室CFU数量的影响 Figure 6 Dose-dependent effect of XL-184 on CFU quantity in the lower chamber. Caco-2 cells were treated with different doses of XL-184 for 24 h and then with equivalent amount of LM for 1.5 h. Cells without XL-184 treatment served as the control. Error bars indicate standard deviations. *P=0.000 vs control group. |
|
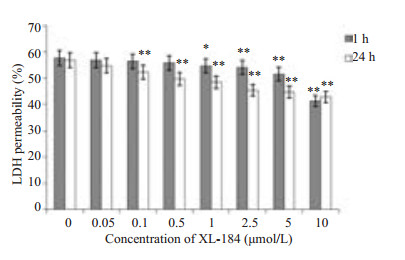
图 7 细胞培养基上清中LDH占总LDH的比例 Figure 7 Percentage LDH in cell culture medium in total LDH. Caco-2 cells were treated with different doses of XL-184 for 1 h and 24 h followed by exposure to equivalent amount of LM (5×107 cfu/ml) for 1.5 h. Cells without XL-184 treatment served as the control. Error bars indicate standard deviations. **P < 0.05, **P < 0.01 vs control group. |
LDH存在于细胞内,当细胞膜受到损伤后,LDH可被释放至培养基中。含XL-184的培养基分别孵育细胞1 h、24 h。两两比较:提前孵育1 h,药物组LDH渗出率低于对照组,药物浓度越高LDH渗出率有下降趋势越明显(P1 < 0.05,P2.5~10 < 0.01)。提前孵育24 h,药物组LDH渗出率低于对照组,并且药物浓度越高LDH渗出率有下降趋势(P0.1~10 < 0.01)。
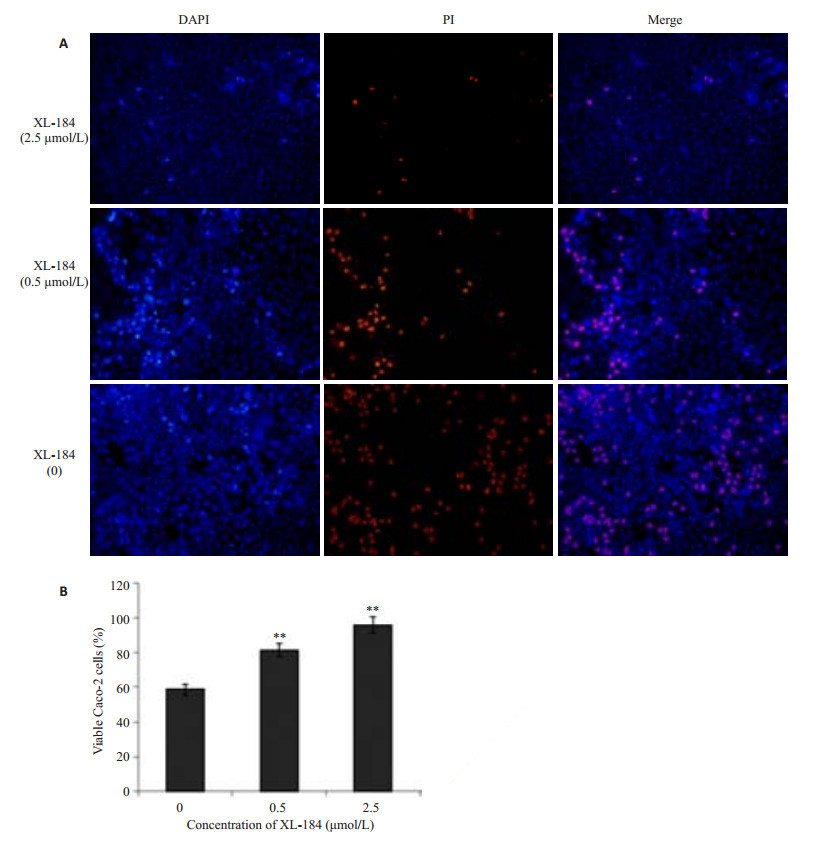
2.7 XL-184对LM所致Caco-2细胞存活率的影响含XL-184浓度为0、0.5、2.5 μmol/L的培养基提前孵育细胞,荧光染色结果(图 8A):不同浓度组细胞存活率具有差异,两两比较(图 8B),药物组细胞存活率大于对照组(P < 0.01)。
|
图 8 不同浓度XL-184对Caco-2细胞存活率的影响 Figure 8 Effect of different concentrations of XL-184 on cell viability in Caco-2 cells. Caco-2 cells were incubated with different doses of XL-184. Caco-2 cell monolayer was incubated with an equivalent amount of LM (5×107 cfu/mL) for 1.5 h. A: PI staining and DAPI staining; B: Statistics of the cell viability. Error bars indicate standard deviations. **P < 0.01 vs control group. |
LM通过内化素B(InlB)模拟肝细胞生长因子或分散因子(HGR或SF),与其受体c-Met结合进入细胞,存在于细胞内的细菌可以逃避机体的免疫防御及清除且抗生素无法进入胞内杀菌,导致LM可在人体内长期存在[18-19]。为了明确c-Met阻断剂XL-184对LM在Caco-2细胞内在化和胞内感染中的抑制效果,本研究应用不同浓度的c-Met受体阻断剂卡博替尼(XL-184)于不同的时间作用Caco-2细胞。由于XL-184与一些药物联合作用可以增强XL-184的效果[20],同时青霉素类抗生素是目前治疗李斯特菌病的首选药物[3-5],因此联合XL-184与氨苄西林抑制LM对Caco-2的感染。结果发现,随着XL-184浓度的升高,LM对Caco-2的侵袭率有明显下降趋势。相同剂量药物作用下,XL-184作用Caco-2细胞24 h即可显著降低LM对Caco-2的侵袭,同时XL-184与氨苄西林联合用药对LM侵袭率降低优于单独使用XL-184或氨苄西林。表明XL-184单独或与氨苄西林联合用药可抑制LM对Caco-2细胞的内在化作用并可预防胞内感染。
研究发现LM感染Caco-2细胞后可降低细胞之间紧密连接的牢固性,并进一步增大细胞的通透性,导致一些小分子物质更容易通过单层细胞[10]。本实验中检测到,在一定的时间内伴随XL-184剂量增大,可显著抑制LM诱导的跨上皮细胞电阻(TEER)值的降低,并可降低HRP和细菌介导的细胞通透性。乳酸脱氢酶(LDH)存在于细胞内,当细胞膜受到破坏后,胞内的LDH释放至培养基中的浓度增高。在本实验中,伴随XL-184作用Caco-2的浓度和时间的增加,细胞释放到胞外的LDH浓度降低,表明XL-184可降低LM诱导的细胞内LDH的释放并进一步降低LM对细胞的损伤。荧光染色方法鉴定Caco-2存活率结果显示,随XL-184剂量增大细胞存活率可显著提高。总之,XL-184可减小LM对Caco-2细胞损伤,且能够增强Caco-2细胞的屏障功能并可以保护LM感染引起的杀细胞效应。
综上所述,针对胞内感染治疗中抗菌药物无法进入细胞内杀菌,且胞内菌具有免疫逃逸作用,致使李斯特菌病成为食传性疾病死亡的重要原因,病死率达30% [21]等棘手问题。本文首次提出c-Met受体阻断剂卡博替尼可以阻断LM侵袭Caco-2,并进一步保护细胞膜的通透性并提高细胞的存活率,同时揭示XL-184与氨苄西林的联合应用可能在更大程度上降低LM对Caco-2的侵袭率。上述结果提示XL-184有望成为一种潜在的预防和治疗LM严重感染的有效药物。XL-184经FDA批准于2012年上市并已完成某些疾病的临床二、三期试验[22-23],本研究可为临床工作者在LM感染的临床治疗中提供新的用药策略。
| [1] |
Giengkam S, Blakes A, Utsahajit PA, et al. Improved quantification, propagation, purification and storage of the obligate intracellular human pathogen orientia tsutsugamushi[J].
PLoS Negl Trop Dis,2015, 9 (8) : e4009.
( 0) 0)
|
| [2] |
Wu S, Wu QP, Zhang JM, et al. Listeria monocytogenes prevalence and characteristics in retail raw foods in china[J].
PLoS One,2015, 10 (8) : e136682.
( 0) 0)
|
| [3] |
徐德民, 武涛, 周美宁. 重症脑膜脑炎型李斯特菌病一例[J].
中国神经免疫学和神经病学杂志,2008, 15 (5) : 392-3.
( 0) 0)
|
| [4] |
Jesus-Alvelo JI. Merenda a.a case report of listeria monocytogenes abscesses presenting as cortically predominant Ring-Enhancing lesions[J].
Case Rep Neurol,2015, 7 (1) : 105-9.
( 0) 0)
|
| [5] |
Williams G, Khan AA, Schweiger F. Listeria meningitis complicating infliximab treatment for Crohn's disease[J].
Can J Infect Dis Med Microbiol,2005, 16 (5) : 289-92.
( 0) 0)
|
| [6] |
Luo X, Cai XE. A combined use of autolysin p60 and listeriolysin O antigens induces high protective immune responses against Listeria monocytogenes infection[J].
Curr Microbiol,2012, 65 (6) : 813-8.
DOI: 10.1007/s00284-012-0238-9. ( 0) 0)
|
| [7] |
Knudsen BS, Woude GV. Showering c-MET-dependent cancers with drugs[J].
Curr Opin Genet Dev,2008, 18 (1) : 87-96.
DOI: 10.1016/j.gde.2008.02.001. ( 0) 0)
|
| [8] |
Pizarro-Cerdá J, Kühbacher A, Cossart P. Entry of Listeria monocytogenes in mammalian epithelial cells: an updated view[J].
Cold Spring Harb Perspect Med,2012, 2 (11) : a010009.
( 0) 0)
|
| [9] |
Torres KE, Zhu QS, Bill K, et al. Activated Met is a molecular prognosticator and potential therapeutic target for malignant peripheral nerve sheath tumors[J].
Clin Cancer Res,2011, 17 (12) : 3943-55.
DOI: 10.1158/1078-0432.CCR-11-0193. ( 0) 0)
|
| [10] |
Koo OK, Amalaradjou MA, Bhunia AK. Recombinant probiotic expressing Listeria adhesion protein attenuates Listeria monocytogenes virulence in vitro[J].
PLoS One,2012, 7 (1) : e29277.
DOI: 10.1371/journal.pone.0029277. ( 0) 0)
|
| [11] |
Tamburro M, Sammarco ML, Ammendolia MG, et al. Evaluation of transcription levels of inlA, inlB, hly, bsh and prfA genes in Listeria monocytogenes strains using quantitative reverse-transcription PCR and ability of invasion into human CaCo-2 cells[J].
FEMS Microbiol Lett,2015, 362 (6) : pii: fnv018.
DOI: 10.1093/femsle/fnv018. ( 0) 0)
|
| [12] |
Sambuy Y, Angelis I, Ranaldi G, et al. The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics[J].
Cell Biol Toxicol,2005, 21 (1) : 1-26.
DOI: 10.1007/s10565-005-0085-6. ( 0) 0)
|
| [13] |
Krieger SE, Kim C, Zhang L, et al. Echovirus 1 entry into polarized Caco-2 cells depends on dynamin, cholesterol, and cellular factors associated with macropinocytosis[J].
J Virol,2013, 87 (16) : 8884-95.
DOI: 10.1128/JVI.03415-12. ( 0) 0)
|
| [14] |
Cajnko MM, Marusic M, Kisovec M, et al. Listeriolysin O affects the permeability of caco-2 monolayer in a Pore-Dependent and Ca2+-Independentmanner[J].
PLoS One,2015, 10 (6) : e130471.
( 0) 0)
|
| [15] |
Cao X, Zhang Y, Zou LY, et al. Persistent oxygen-glucose deprivation induces astrocytic death through two different pathways and calpain-mediated proteolysis of cytoskeletal proteins during astrocytic oncosis[J].
Neurosci Lett,2010, 479 (2) : 118-22.
DOI: 10.1016/j.neulet.2010.05.040. ( 0) 0)
|
| [16] |
Beccia MR, Biver T, Pardini A, et al. The fluorophore 4', 6-diamidino-2-phenylindole(DAPI)induces DNA folding in long double-stranded DNA[J].
Chem Asian J,2012, 7 (8) : 1803-10.
DOI: 10.1002/asia.v7.8. ( 0) 0)
|
| [17] |
Boros-Majewska J, Turczyk L, Wei X, et al. A novel in vitro assay for assessing efficacy and toxicity of antifungals using human leukaemic cells infected with Candida albicans[J].
J Appl Microbiol,2015, 119 (1) : 177-87.
DOI: 10.1111/jam.2015.119.issue-1. ( 0) 0)
|
| [18] |
Almeida MT, Mesquita FS, Cruz RA, et al. Src-dependent tyrosine phosphorylation of non-muscle myosin heavy chain-IIA restricts Listeria monocytogenes cellular infection[J].
J Biol Chem,2015, 290 (13) : 8383-95.
DOI: 10.1074/jbc.M114.591313. ( 0) 0)
|
| [19] |
Schepers K, Dirix V, Mouchet F, et al. Early cellular immune response to a new candidate mycobacterial vaccine antigen in childhood tuberculosis[J].
Vaccine,2015, 33 (8) : 1077-83.
DOI: 10.1016/j.vaccine.2014.12.011. ( 0) 0)
|
| [20] |
Ding L, Zhang ZY, Liang GK, et al. SAHA triggered Met activation contributes to SAHA tolerance in solid cancer cells[J].
Cancer Lett,2015, 356 (2, B) : 828-36.
( 0) 0)
|
| [21] |
Soni DK, Singh DV, Dubey SK. Pregnancy-associated human listeriosis:Virulence and genotypic analysis of Listeria monocytogenes from clinical samples[J].
J Microbiol,2015, 53 (9) : 653-60.
DOI: 10.1007/s12275-015-5243-9. ( 0) 0)
|
| [22] |
Zhang B, Zhang X, Zhou T, et al. Clinical observation of liver cancer patients treated with axitinib and cabozantinib after failed sorafenib treatment: a case report and literature review[J].
Cancer Biol Ther,2015, 16 (2) : 215-8.
DOI: 10.4161/15384047.2014.962318. ( 0) 0)
|
| [23] |
Fallahi P, Ferrari SM, Di Bari FA, et al. Cabozantinib in thyroid cancer[J].
Recent Pat Anticancer Drug Discov,2015, 10 (3) : 259-69.
DOI: 10.2174/1574892810666150708110816. ( 0) 0)
|
 2016, Vol. 36
2016, Vol. 36
