Nasopharyngeal carcinoma(NPC) is one of the most common types of head and neck cancer in Southeast Asia. Currently the prognosis of NPC patients is evaluated based primarily on the Union Internationale Contrele Cancer/American Joint Cancer Committee(UICC/AJCC) TNM staging system. However, there is a discrepancy between the actual clinical outcome and anatomically based TNM stage, indicating that the clinical staging is insufficient for precise prediction of the prognosis[1]. Cancer progression and survival are not determined solely based on the local characteristics of tumor, such as TNM stage, but also involves the host systemic immune/inflammatory response [2]. Nevertheless, the prognostic value of systemic inflammation in patients with NPC has not been sufficiently evaluated.
Measurement of serum albumin and globulin levels and the albumin/globulin ratio(AGR) is routinely performed in clinical laboratories, and they have been exploited as useful prognostic markers for cancer patients. Previous studies demonstrated that a low serum albumin and AGR were independent predictors of poor survival in several types of cancers[3-6], including NPC[7]. Globulin is the major constituent of total serum proteins, and plays a major role in immunity and inflammation. It was reported that a high preoperative globulin level was significantly associated with a poor survival of patients with gastric cancer[8]and rectal cancer[9].
So far the association of globulin level with the prognosis of NPC has not yet been reported. As the measurement of serum globulin is minimally invasive and can be performed before treatment, its prognostic value has raised much interest. In this study, we aimed to assess the prognostic value of serum globulin level before treatment in patients with NPC.
PATIENTS AND METHODS Patient selectionApproval was granted from Medical Ethics Committee of Nanfang Hospital to proceed with this retrospective study, which included 127 patients with newly diagnosed NPC between January, 2009 and December, 2013 at Nanfang Hospital of Southern Medical University. The inclusion criteria were: (a) biopsy-proven primary NPC; (b) pretreatment globulin test; (c) non-disseminated NPC; and(d) receiving definitive radiotherapy at our institute. The exclusion criteria were: (a) simultaneous second primary tumors, (b) chronic inflammatory disease including autoimmune disorder and infection, (c) preexisting liver diseases, and(d) incomplete treatment. The extent of the disease was determined according to the 7th version of UICC/AJCC TNM staging system. Before treatment, all the participants underwent 18F-fluorodeoxyglucose positron emission tomography and computed tomography (18F-FDG PET/CT) to confirm the initial clinical stage.
TreatmentAll the patients were treated with definitive radiotherapy. The radiation dose range was 66-76 Gy in the nasopharynx, 60-70 Gy in the area with positive lymph nodes, and 50-60 Gy in the area of negative lymph nodes. The majority of the patients (83/127) were treated with intensity-modulated radiation therapy(IMRT), and the remaining patients received 3-dimensional conformal radiation therapy(3-DRT). Overall, 16 of the 127 patients(12.6%) were treated with radiotherapy only, and 111(87.4%) received additional platinum-based chemotherapy in line with the National Comprehensive Cancer Network guidelines.
Data CollectionThe clinicopathological characteristics of the patients were recorded at baseline before treatment. All the patients enrolled had serum biochemistry analysis within 14 days prior to any therapeutic intervention. Serum globulin was determined using an automatic biochemical analyzer (Olympus, AU5400, Japan).
Follow-upThe patients were regularly followed up until death or their last visits. They were scheduled to visit the clinics every 3 months in the first 3 years, and every 6 months thereafter. The time of the last follow-up was June, 2015, and the median follow-up duration was 37 months(range 4-75 months). Physical examination and nasopharyngoscopy were performed on each visit. Nasopharyngeal and neck magnetic resonance imaging(MRI), chest X-ray, and abdominal sonogram were performed when clinical indications dictated. Locoregional recurrence was established by biopsy or PET/CT. Distant metastases were diagnosed based on clinical symptoms, physical examination, and imaging methods including plain chest film or CT scan, bone scan, abdominal sonography, or PET/CT scan.
Statistical analysisThe primary endpoint was progression-free survival(PFS), and the secondary endpoints were overall survival(OS), distant metastasis-free survival(DMFS), and locoregional recurrence-free survival(LRFS). PFS was calculated from the first day of treatment to the date of relapse at any site, death or last follow-up. For the remaining endpoints, the duration was measured from the first day of treatment to the date of the target event or censored at the last follow-up date. The receiver-operating characteristic(ROC) curve analysis was performed to select the cut-off point for classifying the pretreatment globulin level as low or high for subsequent analysis. To examine the association of pretreatment serum globulin level with the clinicopathological factors, Chi-square test was used to compare the categorical variables. Survival curves were drawn by the Kaplan-Meier method and compared using log-rank test. Multivariable Cox proportional hazards models were used to test the independent significance. A P values less than 0.05(two-tailed) were considered to indicate a significant difference. All statistical analyses were performed using SPSS version 17.0 (SPSS, Chicago, IL).
RESULTS Patient characteristicsThe clinicopathological characteristics of the 127 patients, including 101(79.5%) male and 26(20.5%) female patients, were presented in Tab. 1. The median age of the patients at diagnosis was 44 years(range 17-76 years). Based on the World Health Organization(WHO) criteria, 92.9% of the patients had type IIa or IIb disease and 7.1% had type I disease.
| Table 1 Patient characteristics |
During the follow-up of the 127 patients, distant metastasis occurred in 19, locoregional recurrence in 7, and both distant metastasis and locoregional recurrence in 3 patients; death occurred in 13 cases. The overall 3-year PFS, 3-year OS, 3-year DMFS, and 3-year LRFS in these patients were 79.1%, 89.5%, 83.4%, and 95.6%, respectively. Twelve patients were lost to the follow-up.
Optimal cut-off value for defining low or high globulin levelThe mean pretreatment serum globulin level was 28.09 g/L(range 19.50-42.20 g/L) in these patients. As PFS was the primary endpoint, we selected the values that showed the best trade-off between sensitivity and specificity for PFS as the cut-off value for pretreatment serum globulin level. As determined by ROC analysis(Fig. 1), the optimal cut-off value of pretreatment serum globulin level was 30.05 g/L, by which the patients were divided into low(≤30.05 g/L) or high ( > 30.05 g/L) globulin group.
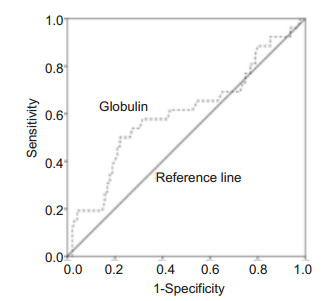
|
Figure 1 Receiver-operating characteristic (ROC) curve for pretreatment serum globulin level as a predictor of progression in 127 nasopharyngeal carcinoma (NPC) patients. The cut-off value of globulin was 30.05 g/L. The area under the ROC curve (AUC) was 0.6. |
Based on the optimal cut-off value, 91/127 patients(71.7%) had low globulin levels and 36/127(28.3%) had high globulin levels before the treatment. The distribution of the pretreatment serum globulin level differed significantly when the patients were stratified by gender and N stage(Tab. 1). A significantly greater proportion of male patients was found in low globulin group(77/91, 76.2%) than in high globulin group(24/ 36, 23.8%) (P=0.024). In addition, there were more N2-3b stage patients in high globulin group than in low globulin group(P=0.016). No significant association was found between pretreatment serum globulin level and the other clinicopathological parameters.
Prognostic value of pretreatment serum globulin level in NPCKaplan-Meier analysis identified pretreatment serum globulin level as a statistically significant predictive factor for PFS(Fig. 2A), OS(Fig. 2B), and DMFS(Fig. 2C). Compared with patients in low globulin group, the patients in high globulin group had a significantly higher 3-year PFS (82.5% vs 70.4%; P=0.019). In addition, patients with low globulin levels had a better 3-year OS than those with high globulin levels(92.6% vs 81.2%; P=0.034). Furthermore, the 3-year DMFS rates differed significantly between patients with low and high pretreatment serum globulin levels(86.2% vs 76.4%, P=0.049). However, pretreatment serum globulin levels had no prognostic value for LRFS(P=0.114; Fig. 2D), probably because of the small sample size of the patients with locoregional recurrence.
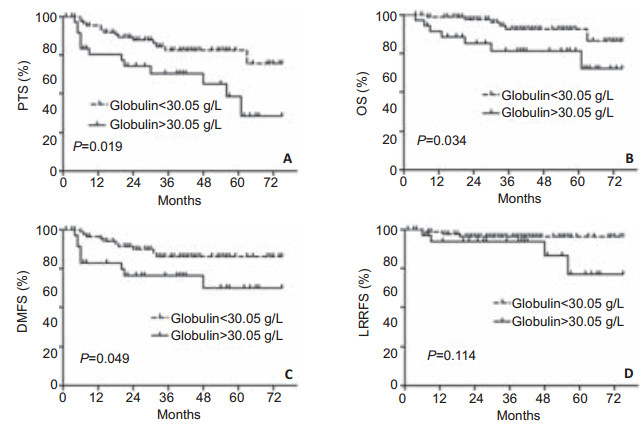
|
Figure 2 Kaplan-Meier analysis to estimate the probabilities of survival for patients stratified by pretreatment serum globulin level. A: Progression-free survival(PFS); B: Overall survival(OS); C: Distant metastasis-free survival(DMFS); D: Locoregional recurrence-free survival(LRFS). |
Multivariate analysis for PFS, OS and DMFS was performed to adjust for various prognostic factors. The following parameters were included in the Cox proportional hazards model: age(≤44 vs > 44 years), gender(male vs female), T classification(T1-2 vs T3-4), N classification (N0-1 vs N2-3b), and pretreatment globulin(≤30.05 g/L vs > 30.05 g/L). A high pretreatment serum globulin level remains the independent prognostic factor for a poor PFS(HR=2.344, P=0.031) but not for OS(P=0.060) or DMFS(P=0.090). N stage was an independent predictive factor for PFS(P=0.012) and OS(P=0.027). Age was also identified as an independent predictive factor for OS(P=0.014) in multivariate analysis(Tab. 2).
| Table 2 Multivariate Cox regression analysis |
NPC treatment remains challenging because of a high tendency towards relapse, especially distant metastasis. The identification of prognostic factors for NPC is important for risk stratification and potential improvement of the treatment outcomes. It is especially beneficial if it is achieved noninvasively. To our knowledge, this is the first report of the association of pretreatment serum globulin level with the prognosis and clinicopathological parameters in NPC.
Serum globulin is produced by the human monocyte-phagocyte system and maintains osmotic pressure, buffers blood pH, and plays a role in the transport of hormones, fatty acids, and other compounds. High levels of globulins are results of an increased accumulation of acute-phase proteins and immunoglobulins as well as other serum proteins; these changes are signs of an inflammatory state. Previous studies reported a significant association of low globulin levels with favorable survival in gastric cancer[8]and rectal cancer[9]. Du et al[7] investigated the association of albumin and AGR with the prognosis in NPC, but they did not evaluate the predictive value of globulin. In this study, we found that globulin was significantly associated with PFS, OS, and DMFS in NPC. Although globulin was associated with gender and N stage, the predictive value of pretreatment serum globulin level for PFS remained significant after adjustment for gender, N stage and other clinical characteristics in multivariate analysis.
It has been suggested that cancer-related inflammation represents a hallmark of malignant tumors [10, 11]. In-flammatory responses lead to chronic oxidative stress and the generation of oxygen free radicals, which have been shown to stimulate cancer initiation, promotion, and progression [12]. Systemic inflammation may also protect circulating metastatic cancer cells from NK cell-mediated killing, thereby overcoming immunosur-veillance[12]. Neutrophils in peripheral blood or in tumor microenvironment have been shown to produce proangiogenic factors(including vascular endothelial growth factor) to stimulate tumor development[13, 14]. With the development of tumor biology, accumulating evidence shows that systemic inflammatory response is associated with a poor survival in patients with various cancers[15-19]. C-reactive protein(CRP) is mainly synthesized and released into the systemic circulation by hepatocytes, and is used as a non-specific marker of inflammation. A significantly increased CRP level is associated with a poor survival in NPC, particularly in patients in an advanced stage[20, 21]. A high neutrophil to lymphocyte ratio is also associated with a poor survival in patients with NPC[22, 23].
Why is cancer-related inflammation especially important in NPC? First, inflammation is a key component of the tumor microenvironment. A consistent, massive leukocytic infiltration is present in virtually all primary tumors, and is suspected to enhance the malignant growth of NPC cells [24]. Second, Epstein-Barr viral(EBV) infection is associated with NPC in the areas
where NPC is endemic, and induces a consistent expression of viral immunogenic proteins that leads to a potent immune response[24, 25]. EBV-encoded RNAs(EBERs) cause chronic inflammation, and play a pivotal role in inflammation-to-oncogenesis transition in NPC development[26, 27]. Finally, high levels of numerous cytokines and other inflammation-related factors are consistently detected in the peripheral blood of patients with NPC[24].
It is now recognized that disease progression incancer patients is determined not only by tumor characteristics, but also by the host inflammatory response. Cancer-related inflammation represents a target for innovative therapeutic strategies. For many years, all efforts to treat cancer have focused on the destruction/ inhibition of tumor cells. Strategies to modulate the host microenvironment offer a complementary perspective. Primary proinflammatory cytokines represent the prime targets and ongoing results in this direction justify continuing efforts[28, 29]. Anti-inflammatory therapy in a variety of cancers has become a research hotspot. Several anti-inflammatory drugs have been found to reduce tumor incidence when used as prophylactics, and can slow down progression and reduce mortality when used as therapeutics, particularly in the case of sporadic colon cancer[30]. Such drugs include COX2 inhibitors, aspirin, and anti-inflammatory steroids(such as dexamethasone). In addition to its well-documented preventive effects in colon cancer, aspirin also reduces the incidence of breast cancer[31] and lowers the risk of prostate cancer in individuals that carry a particular polymorphic allele at the lymphotoxin a locus, which specifies high lymphotoxin production[32]. Recently, it has been reported that nonsteroidal anti-inflammatory drugs, but not aspirin, are associated with a reduction in the risk of bladder cancer [33] and urothelial carcinoma [34]. The value of anti-inflammatory therapy in NPC needs to be explored further.
The principal limitations of this study are its retrospective nature, insufficient follow-up for some patients and inclusion of the patients from a single institution. Moreover, 92.9% of patients had WHO IIa-b disease and 7.1% had WHO I disease in our study, which may be slightly different from previous research data, probably because of the small sample size without a stratified analysis. A multicenter prospective design is required for further validation of our finding.
ConclusionIn summary, an elevated serum globulin before treatment predicts a poor prognosis in patients with NPC. Pretreatment globulin level may serve as a valuable marker for stratifying patients and individualized therapy. Further studies are needed to clarify whether the combined assessment of tumor characteristics and host systemic inflammatory status can improve risk stratification in NPC.
| [1] |
Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection[J].
Nat Rev Cancer,2005, 5 (11) : 845-56.
DOI: 10.1038/nrc1739. ( 0) 0)
|
| [2] |
Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development[J].
Mol Cancer Res,2006, 4 (4) : 221-33.
DOI: 10.1158/1541-7786.MCR-05-0261. ( 0) 0)
|
| [3] |
Duran AO, Inanc M, Karaca H, et al. Albumin-globulin ratio for prediction of long-term mortality in lung adenocarcinoma patients[J].
Asian Pac J Cancer Prev,2014, 15 (15) : 6449-53.
DOI: 10.7314/APJCP.2014.15.15.6449. ( 0) 0)
|
| [4] |
Yao Y, Zhao M, Yuan D, et al. Elevated pretreatment serum globulin albumin ratio predicts poor prognosis for advanced non-small cell lung cancer patients[J].
J Thorac Dis,2014, 6 (9) : 1261-70.
( 0) 0)
|
| [5] |
Azab B, Kedia S, Shah N, et al. The value of the pretreatment albumin/globulin ratio in predicting the long-term survival in colorectal cancer[J].
Int J Colorectal Dis,2013, 28 (12) : 1629-36.
DOI: 10.1007/s00384-013-1748-z. ( 0) 0)
|
| [6] |
Azab BN, Bhatt VR, Vonfrolio S, et al. Value of the pretreatment albumin to globulin ratio in predicting long-term mortality in breast cancer patients[J].
Am J Surg,2013, 206 (5) : 764-70.
DOI: 10.1016/j.amjsurg.2013.03.007. ( 0) 0)
|
| [7] |
Du XJ, Tang LL, Mao YP, et al. The pretreatment albumin to globulin ratio has predictive value for long-term mortality in nasopharyngeal carcinoma[J].
PLoS One,2014, 9 (4) : e94473.
DOI: 10.1371/journal.pone.0094473. ( 0) 0)
|
| [8] |
Chen J, Zhou Y, Xu Y, et al. Low pretreatment serum globulin may predict favorable prognosis for gastric cancer patients[J].
Tumour Biol,2015 .
DOI: 10.1007/s13277-015-3778-3. ( 0) 0)
|
| [9] |
Li Q, Meng X, Liang L, et al. High preoperative serum globulin in rectal cancer treated with neoadjunctive chemoradiation therapy is a risk factor for poor outcome[J].
Am J Cancer Res,2015, 5 (9) : 2856-64.
( 0) 0)
|
| [10] |
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation[J].
Cell,2011, 144 (5) : 646-74.
DOI: 10.1016/j.cell.2011.02.013. ( 0) 0)
|
| [11] |
Colotta F, Allavena P, Sica A, et al. Cancer-related inflammation, the http://www[J].
Carcinogenesis,2009, 30 (7) : 1073-81.
DOI: 10.1093/carcin/bgp127. ( 0) 0)
|
| [12] |
Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer[J].
Cell,2010, 140 (6) : 883-99.
DOI: 10.1016/j.cell.2010.01.025. ( 0) 0)
|
| [13] |
Kusumanto YH, Dam WA, Hospers GA, et al. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor[J].
Angiogenesis,2003, 6 (4) : 283-87.
DOI: 10.1023/B:AGEN.0000029415.62384.ba. ( 0) 0)
|
| [14] |
Fondevila C, Metges JP, Fuster J, et al. p53 and VEGF expression are independent predictors of tumour recurrence and survival following curative resection of gastric cancer[J].
Br J Cancer,2004, 90 (1) : 206-15.
DOI: 10.1038/sj.bjc.6601455. ( 0) 0)
|
| [15] |
Park JH, Watt DG, Roxburgh CS, et al. Colorectal cancer, systemic inflammation, and outcome: staging the tumor and staging the host[J].
Ann Surg,2015, 263 (2) : 326-36.
DOI: 10.1097/SLA.0000000000001122. ( 0) 0)
|
| [16] |
Shao N, Cai Q. High pretreatment neutrophil-lymphocyte ratio predicts recurrence and poor prognosis for combined small cell lung cancer[J].
Clin Transl Oncol,2015, 17 (10) : 772-78.
DOI: 10.1007/s12094-015-1289-8. ( 0) 0)
|
| [17] |
Maeda K, Shibutani M, Otani H, et al. Inflammation-based factors and prognosis in patients with colorectal cancer[J].
World J Gastrointest Oncol,2015, 7 (8) : 111-17.
DOI: 10.4251/wjgo.v7.i8.111. ( 0) 0)
|
| [18] |
Pinato DJ. Cancer-related inflammation: an emerging prognostic domain in metastatic castration-resistant prostate carcinoma[J].
Cancer,2014, 120 (21) : 3272-74.
DOI: 10.1002/cncr.28889. ( 0) 0)
|
| [19] |
Hong J, Mao Y, Chen X, et al. Elevated preoperative neutrophilto-lymphocyte ratio predicts poor disease-free survival in Chinese women with breast cancer[J].
Tumour Biol,2015 .
DOI: 10.1007/s13277-015-4233-1. ( 0) 0)
|
| [20] |
Zeng YC, Wu R, Xiao YP, et al. Serum C-reactive protein predicts poor prognosis in patients with locoregionally advanced nasopharyngeal carcinoma treated with chemoradiotherapy[J].
Curr Oncol,2015, 22 (1) : 20-24.
( 0) 0)
|
| [21] |
Xia WX, Zhang HB, Shi JL, et al. A prognostic model predicts the risk of distant metastasis and death for patients with nasopharyngeal carcinoma based on pre-treatment serum C-reactive protein and N-classification[J].
Eur J Cancer,2013, 49 (9) : 2152-60.
DOI: 10.1016/j.ejca.2013.03.003. ( 0) 0)
|
| [22] |
An X, Ding PR, Wang FH, et al. Elevated neutrophil to lymphocyte ratio predicts poor prognosis in nasopharyngeal carcinoma[J].
Tumour Biol,2011, 32 (2) : 317-24.
DOI: 10.1007/s13277-010-0124-7. ( 0) 0)
|
| [23] |
He JR, Shen GP, Ren ZF, et al. Pretreatment levels of peripheral neutrophils and lymphocytes as independent prognostic factors in patients with nasopharyngeal carcinoma[J].
Head Neck,2012, 34 (12) : 1769-76.
DOI: 10.1002/hed.v34.12. ( 0) 0)
|
| [24] |
Gourzones C, Barjon C, Busson P. Host-tumor interactions in nasopharyngeal carcinomas[J].
Semin Cancer Biol,2012, 22 (2) : 127-36.
DOI: 10.1016/j.semcancer.2012.01.002. ( 0) 0)
|
| [25] |
Lin JC, Wang WY, Chen KY, et al. Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma[J].
N Engl J Med,2004, 350 (24) : 2461-70.
DOI: 10.1056/NEJMoa032260. ( 0) 0)
|
| [26] |
Duan Y, Li Z, Cheng S, et al. Nasopharyngeal carcinoma progression is mediated by EBER-triggered inflammation via the RIG-I pathway[J].
Cancer Lett,2015, 361 (1) : 67-74.
DOI: 10.1016/j.canlet.2015.02.037. ( 0) 0)
|
| [27] |
Li Z, Duan Y, Cheng S, et al. EBV-encoded RNA via TLR3 induces inflammation in nasopharyngeal carcinoma[J].
Oncotarget,2015, 6 (27) : 24291-303.
DOI: 10.18632/oncotarget. ( 0) 0)
|
| [28] |
Loberg RD, Ying C, Craig M, et al. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo[J].
Cancer Res,2007, 67 (19) : 9417-24.
DOI: 10.1158/0008-5472.CAN-07-1286. ( 0) 0)
|
| [29] |
Harrison ML, Obermueller E, Maisey NR, et al. Tumor necrosis factor alpha as a new target for renal cell carcinoma: two sequential phase II trials of infliximab at standard and high dose[J].
J Clin Oncol,2007, 25 (29) : 4542-49.
DOI: 10.1200/JCO.2007.11.2136. ( 0) 0)
|
| [30] |
Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2[J].
Nat Rev Cancer,2001, 1 (1) : 11-21.
DOI: 10.1038/35094017. ( 0) 0)
|
| [31] |
Gierach GL, Lacey JJ, Schatzkin A, et al. Nonsteroidal antiinflammatory drugs and breast cancer risk in the national institutes of Health-AARP diet and health study[J].
Breast Cancer Res,2008, 10 (2) : R38.
DOI: 10.1186/bcr2089. ( 0) 0)
|
| [32] |
Liu X, Plummer SJ, Nock NL, et al. Nonsteroidal antiinflammatory drugs and decreased risk of advanced prostate cancer: modification by lymphotoxin alpha[J].
Am J Epidemiol,2006, 164 (10) : 984-89.
DOI: 10.1093/aje/kwj294. ( 0) 0)
|
| [33] |
Daugherty SE, Pfeiffer RM, Sigurdson AJ, et al. Nonsteroidal antiinflammatory drugs and bladder cancer: a pooled analysis[J].
Am J Epidemiol,2011, 173 (7) : 721-30.
DOI: 10.1093/aje/kwq437. ( 0) 0)
|
| [34] |
Shih C, Hotaling JM, Wright JL, et al. Long-term NSAID use and incident urothelial cell carcinoma in the VITamins and Lifestyle (VITAL) study[J].
Urol Oncol,2013, 31 (8) : 1689-95.
DOI: 10.1016/j.urolonc.2012.06.001. ( 0) 0)
|
 2016, Vol. 36
2016, Vol. 36
