2. 江西省儿童医院,江西 南昌 330006
2. Jiangxi Provincial Children's Hospital,Nanchang 330006,China
鼠伤寒沙门菌(STM)为食源性致病菌,属于革兰氏阴性菌,主要通过食入受污染的食物或水感染人类、家畜及啮齿类特异性宿主。沙门氏菌感染引起以单核吞噬细胞系统增生性改变为病理特征的急性胃肠炎,部分可侵入肠上皮细胞、吞噬细胞,经内化后可继续播散,导致食物中毒、败血症、休克等危及生命的肠道外系统性疾病,甚至会引起妊娠并发症,如绒毛膜羊膜炎经胎盘感染而致流产发生[1-3]。沙门菌病的发病率和致死率均位于细菌性疾病的前列,全球每年感染人数可能超过90万,其中5%的人口受这种潜在的致命性全身性疾病的威胁[4-6]。随着经济水平的提高,对供水和食品卫生要求的提高,我国对伤寒的感染有一定控制,但在贵州、浙江、安徽等省仍时有爆发流行。近年来发生多次重大自然灾害,沙门菌被列为灾后重点防治的传染病[7]据统计,澳大利亚在沙门菌病的治疗和预防的花费每年高达12亿美元[8]。由于消灭沙门菌的希望不太,反而随着多重耐药沙门菌的出现,使沙门菌病的防治变得更有难度[9]。目前,沙门菌仍然是危害人类健康、畜牧业发展的人畜共患病原体之一[10-11]。
沙门菌需要染色体和质粒上多种毒力因子共同作用才致病。沙门菌大质粒已成为目前的研究热点。Kurita等报道,沙门菌质粒毒力基因(spv)是高度保守序列,是导致全身严重系统性疾病必不可少的毒力[9, 12]。其中,spvB和spvC是spv结构基因中对细菌毒力最强的基因[9],Matsui等[13]认为spvB和spvC基因足以取代spv介导的毒力,但spvB、spvC基因之间是否存在相关性及其确切致病机制尚未阐明。
根据“分子Koch'S原则”,微生物中的某基因是否与感染性疾病的存在有因果关系,验证的最佳方法是采用突变体分析[14]。本研究通过沙门菌不同表型的缺陷变异株来研究spvB、spvC基因对沙门菌毒力及对宿主免疫系统的影响,及两毒力基因间可能存在的相关性,并为沙门菌减毒疫苗的制备奠定基础。
1 材料与方法 1.1 实验材料 1.1.1 细菌携带spv基因的野生型鼠伤寒沙门菌株211(STM.211)是由江西省儿童医院微生物室保存的临床分离株;敲除spvB 基因的沙门菌211(STM.211-ΔspvB)、敲除spvC基因的沙门菌211(STM.211-ΔspvC)、spvB.spvC基因共同缺陷的沙门菌211(STM.211-ΔspvB.spvC)由本实验组参考基因敲除相关文献[15-17]并进行改良在前期实验完成,-80 ℃保存。
1.1.2 动物125只BALB/c小鼠,6周龄,均为雌性,体质量:17.40±1.23 g,购自江西中医学院。
1.1.3 主要试剂、设备及器材Mouse IL-10 ELISA Kit、 Mouse IL-12 ELISA Kit、Mouse IFN-γ ELISA KitRayBIO®;甲醛(西陇化工股份有限公司);超低温冰箱(德国西门子公司);CO2培养箱(BSD-100,上海博讯);分光光度计(752C,上海第三分析仪器厂);酶标仪(芬兰MK-3);显微镜(Nikon ECLIPSE 80i),显微数码相机(Nikon DS-Ri2);医用超净工作台(YJ一875A,吴江市净化设备总厂)。
1.2 实验方法 1.2.1 模型建立(1)动物饲养:BALB/c小鼠125只,笼间消化道隔离,自由进食、水,室温20~22 ℃,环境湿度50%~55%;(2)分组:完全随机分组法将小鼠分为5组,分别为STM.211 组、STM.211-ΔspvB 组、STM.211-ΔspvC组、STM.211-ΔspvB.spvC组、PBS组,PBS组为阴性对照组,每组25只;(3)细菌培养、定量:将储存在-80 ℃冰箱里的STM.211、STM.211-ΔspvB、STM.211-ΔspvC、STM.211-ΔspvB.spvC复苏,分别接种于2 mL LB培养液中,37 ℃ 250 r/min过夜培养后接种至SS培养皿中,置于37 ℃ CO2培养箱中过夜培养,次日分别挑取单菌落鉴定后接种在SS培养皿上,保存待用。用前挑取单个菌落增菌使用。
细菌定量:将增菌后的实验菌,采用10-1级(即用0.3 mL菌液加至2.7 mL LB中混匀)连续稀释,使用分光光度计测定在D600下菌液的光密度值,直到实验所需要的浓度。同时做菌落培养计数,所得结果与分光光度计测得的值相符;(4)制备BALB/c小鼠感染模型:挑取实验菌接种于LB 液体培养基中,调整细菌浓度约2×105 CFU/mL,备用。除PBS组经i.p. 0.2 mL PBS外,余各组均分别经i.p. 0.2 mL 2×105 CFU/mL相对应的实验菌,每天观察小鼠精神状态、大便情况、体质量、毛发色泽变化等情况。根据预实验结果,分别在感染后1、3、5、7、9 d各组随机取5只小鼠经摘眼球取血后颈椎脱臼法处死小鼠,将收集的血液置于室温中3~4 h,于4 ℃恒温离心机中4000 r/min 离心4 min,吸取上清,置于-80 ℃冰箱保存待测,并分离小鼠肝脏和脾脏进行后续实验。
1.2.2 小鼠肝、脾脏器的病理切片解剖小鼠分离肝脏和脾脏,用生理盐水清洗标本表面血液、粘液,取体积约为2.0 cm×2.0 cm×0.3 cm的脏器标本置于40%甲醛溶液中固定;材料经固定后流水冲洗数小时,材料依次经70%、80%、90%浓度的乙醇脱水30 min,再放入95%、100%各2次,每次20 min;透明剂二甲苯处理后石腊包埋,切片并用苏木精和伊红染色(HE染色)、封片,光镜下观察脏器病理变化。
1.2.3 ELISA 法检测血清细胞因子分别按照MouseIL-10 ELISA Kit、Mouse IL-12 ELISA Kit 及MouseIFN-γ ELISA Kit试剂盒说明操作:(1)用前将所有的试剂及样本置于室温(18~25 ℃)中;(2)将100 μL各样本加入对应的孔板中,各做3个复孔,另作空白孔对照,覆盖孔板并在室温中轻轻摇动孵育2.5 h;(3)弃去溶液,用排抢将300 μL 1×洗涤液填满孔板洗涤4次;使用吸水纸将洗涤液彻底去除干净;(4)每孔中加100 μL生物素标记的单抗(IL-10、IL-12 或IFN-γ),覆盖孔板并在室温中轻轻摇动孵育1 h;(5)弃去溶液,重复步骤三;(6)每孔中加100 μL链霉抗生物素蛋白溶液,覆盖孔板并在室温中轻轻摇动孵育45 min;(7)弃去溶液,重复步骤三;(8)每孔中加100 μL TMB一步法底物试剂,覆盖孔板,在室温中轻轻摇动避光孵育30 min;(9)每孔中加入50 μL终止液,底物液体变蓝,立即在酶标仪D450 nm上检测。
1.3 数据分析所有数据均用SPSS 17.0软件进行统计分析;数据用均数±标准差表示,实验各组间时点先做单因素组内差分析,符合方差齐性的用LSD检验作两两比较,不满足方差齐性的用Dunnett's T3检验作两两比较。检验水准设定α=0.05,则P<0.05差异有统计学意义。
2 结果 2.1 感染后小鼠的感染中毒症状感染沙门菌后1 d起,野生菌株组和突变株组小鼠均有精神状态差,活动少、聚集蜷缩,进食有减少,毛发疏松、体质量不增等现象,部分小鼠发生激惹症状,这些症状以野生均株为重,且以感染沙门菌后3~5 d最为明显,但各组均无腹泻症状;PBS组小鼠精神状态良好,毛发顺,活动如常,进食良好,生长良好。按中毒症状的严重程度排序:STM.211>STM.211-ΔspvB、STM.211-spvCSTM.211-ΔspvB.spvC>PBS。
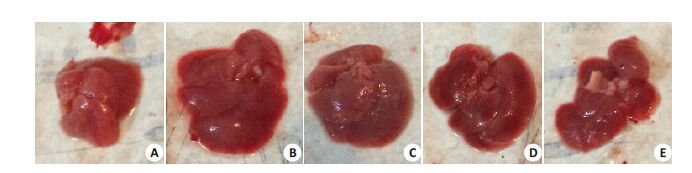
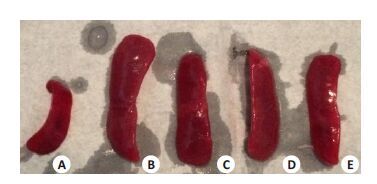
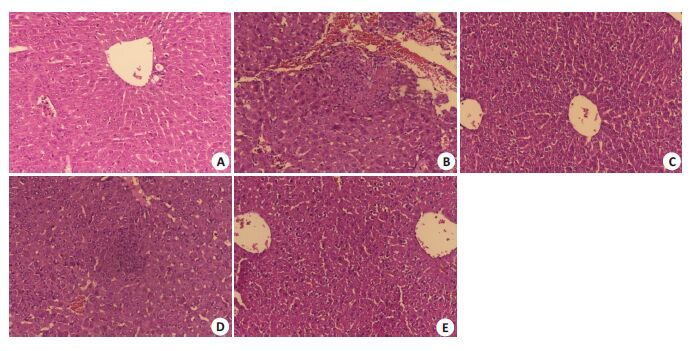
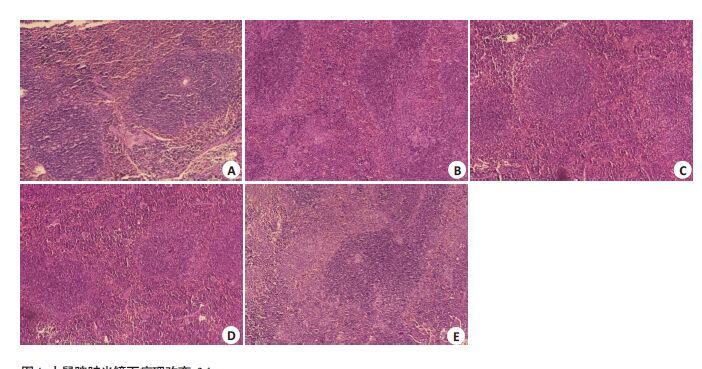
2.2 小鼠肝脏和脾脏的病理变化感染沙门菌后1 d,各组间肝脾饱满有光泽,大小与正常对照组无明显差异。感染后3 d起,分离小鼠肝脾,肉眼观察发现感染沙门菌的小鼠的肝脾明显增大并充血,肝脏表面出现点状坏死病灶,光镜下观察可见肝细胞呈水样变性,汇管区炎性细胞浸润及点片状坏死灶;脾脏可见多核巨细胞增生,皮质和髓质分界不清,脾窦明显扩张充血。上述病理改变均以野生型沙门菌感染组最为明显,而STM.211-ΔspvB、STM.211-ΔspvC次之,STM.211-ΔspvB.spvC组最轻(图 1~4)。
|
图 1 小鼠肝脏大体观改变 Figure 1 General view changes of the liver (3 d). A: PBS; B: STM.211; C: STM.211-ΔspvB; D: STM.211-ΔspvC; E:STM.211-ΔspvB.spvC. The liver of mice which infected with salmonella are increased and congestion, and dotted necroticlesions. The degree of the pathological changes: STM.211>STM.211-ΔspvB、STM.211-ΔspvC>SSTM.211-ΔspvB.spvC. |
|
图 2 小鼠脾脏大体观改变 Figure 2 General view changes of the spleen (3 d). A: PBS; B:STM.211; C: STM.211-ΔspvB; D: STM.211-ΔspvC; E:STM.211-ΔspvB.spvC. The spleen of mice which infected withsalmonella are increased and congestion.The degree of thepathological changes: STM.211>STM.211-ΔspvB、STM.211-ΔspvC>STM.211-ΔspvB.spvC. |
|
图 3 小鼠肝脏光镜下病理改变 Figure 3 Light microscopy view changes of the liver, 3 d(HE, Original magnification: × 40). A: PBS; B: STM.211; C: STM.211-ΔspvB; D: STM.211-ΔspvC; E: STM.211-ΔspvB.spvC. The hepatocyte is hydropic degeneration, and its collect abbacyinflammatory cells infiltration and focal point of flake necrosis. The degree of the pathological changes: STM.211>STM.211-ΔspvB、STM.211-ΔspvC>STM.211-ΔspvB.spvC. |
|
图 4 小鼠脾脏光镜下病理改变,3d Figure 4 Light microscopy view changes of the spleen, 3 d(HE, Original magnification: × 20). A: PBS; B: STM.211; C:STM.211-ΔspvB; D: STM.211-ΔspvC; E: STM.211-ΔspvB.spvC. The spleen have multinucleated giant cell hyperplasia,and thereis no clear boundary between cortex and medulla,splenic sinus expansion hyperemia. The degree of the pathologicalchanges: STM.211>STM.211-ΔspvB、STM.211-ΔspvC>STM.211-ΔspvB.spvC. |
感染沙门菌后,小鼠血清中IFN-γ和IL-12分泌量显著增加,而IL-10 升高的程度相对较低,在3 d时达高峰,STM.211、STM.211-ΔspvB、STM.211-ΔspvC 和STM.211-ΔspvB.spvC 组IL-10、IL-12 和IFN-γ 均高于PBS组(阴性对照组)(P<0.05);但不同菌株感染组间细胞因子分泌存在差异:STM.211、STM.211-ΔspvB、STM.211-ΔspvC 组IFN-γ和IL-12 分泌量均明显低于STM.211-ΔspvB.spvC组(P<0.05),而IL-10的分泌量却高于STM.211-ΔspvB.spvC 组(P<0.05);但是STM.211、STM.211-ΔspvB、STM.211-ΔspvC组的组间差异无统计学意义(P>0.05,表 1)。
| 表 1 血清细胞因子水平 Table 1 Serum cytokine levels (pg/mL, Mean±SD, n=5) |
为逃避宿主免疫防御,沙门菌通过SPI 编码的T3SS分泌系统(T3SSs)释放多种效应蛋白至宿主细胞抵抗宿主的免疫反应。spvB是对细菌毒力影响较大的结构基因,其编码的蛋白经T3SS-2系统导入宿主细胞,诱导肌动蛋白解聚,使受染后细胞发生凋亡[18],并且与肠系膜淋巴结、脾、肝等肠道外组织严重播散性感染有关[19-20]。spvC蛋白可抑制MAPKs,将EPK1/2去磷酸化,阻止一些转录因子从细胞质转至细胞核,起到减少肠道炎症的作用。这导致沙门菌逃避了肠粘膜的免疫应答,有利于沙门菌在感染初始部位生长繁殖,引起全身感染。沙门菌SopB、SigD 蛋白激活AKT蛋白激酶,抑制宿主细胞死亡,促使沙门菌发生持续性感染[21-22]。
胞内菌(如沙门菌、Listeria菌和分支杆菌)可促使抗原活化的初始性CD4+T细胞(TH0)分化成TH1效应细胞,通过T细胞表面CD40L与APC表面CD40 相互作用,促进APC产生IL-12。IL-12p40具有启动适应性免疫应答的作用,可诱导TH0 向TH1 转化,并对胞内菌、寄生虫感染有保护性作用[23-24]。而缺乏IL-12/ IL-12受体者对沙门菌的易感性明显升高,提示IL-12是胞内菌的免疫应答中一个重要的细胞因子[25-26]。这也许与IL-12与p40促使更多的巨噬细胞活化,进一步加强了固有免疫反应水平有关[27]。沙门菌为胞内感染菌,其免疫应答主要以T细胞为主的细胞免疫。IL-12减少,其介导的抗胞内菌的细胞免疫功能下降,沙门菌可在宿主细胞中生长繁殖,甚至向其他组织器官扩散。
IL-12还诱导单核细胞、巨噬细胞和树突状细胞释放IFN-γ,通过细胞因子介导的巨噬细胞活化,释放颗粒酶和穿孔素使靶细胞溶解来清除胞内菌[28]。在抗胞内菌过程中,IFN-γ诱导增强氧化杀伤作用,提高杀灭胞内菌的能力,此外,IFN-γ还可促进多种细胞MHCⅠ类和Ⅱ类分子的表达;促进TH1细胞分化、抑制TH2细胞;抗病毒;参与炎性反应等。活化的巨噬细胞促进吞噬体成熟,酸化液泡,产生活性氮中间体(RNIS)、一氧化氮合酶2(NOS2)、抗菌肽和三磷酸酶蛋白LRG-47等抗微生物分子,阻止细菌复制[29-30]。IFN-γ可经刺激巨噬细胞产生IL-12,与IL-12共同诱导TH0细胞向TH1细胞分化,增强细胞免疫。缺乏IFN-γ有利于细菌繁殖,使宿主易感性增加[31]。本研究发现,STM.211、STM.211- ΔspvB、STM.211-ΔspvC 组之间小鼠IL-12 无明显差别(P<0.05),但较STM.211-ΔspvB.spvC 更低(P<0.05),可见,spvB/spvC基因可使小鼠血清中IL-12分泌减少,加重靶器官病理改变
IL-10作为重要的抗炎介质,能抑制多种促炎因子的产生和Th1细胞应答,参与Th2型细胞介导的抗炎反应。研究发现,结核分枝杆菌和利什曼虫的持续性感染需要IL-10支持,这表明,IL-10在胞内菌的持续性感染中发挥重大作用[32]。诱导IL-10产生可能是病原体逃避TH1细胞的免疫应答,促进TH2细胞表达的一种机制[25, 33]。
本研究中发现STM.211、STM.211-ΔspvB 和STM.211-ΔspvC 菌株感染的小鼠的中毒症状明显较PBS组严重,STM.211-ΔspvB.spvC组与PBS组间无明显差异。沙门菌感染后,IFN-γ和IL-12分泌量显著增高,而IL-10 升高的程度较低,产生以Th1优势应答为主的免疫反应,但不同菌株间导致细胞因子的分泌存在差异,STM.211组、STM.211-ΔspvB组、STM.211-ΔspvC组的IFN-γ和IL-12 分泌量均显著低于STM.211-ΔspvB.spvC 组(P<0.05),而IL-10的分泌量明显高于STM.211-ΔspvB.spvC 组(P<0.05)。细胞因子具有调节免疫的生物学活性,是决定巨噬细胞功能表型的决定性因素。这项研究提示,沙门菌spvB、spvC均能影响宿主分泌细胞因子的水平,抑制Th1 细胞因子IL-12 和IFN-γ,促进Th2细胞因子IL-10的表达,使免疫应答向TH2方向偏移。可见,spvB或(和)spvC基因具有转换不同免疫应答类型的功能,从而有利于宿主菌抵抗和逃避宿主的免疫防御。此外,spvB 或(和)spvC 基因均可增加沙门菌毒力,加重宿主感染结局。但STM.211(spvB+spvC+)组、STM.211-ΔspvB组、STM.211-ΔspvC组间差异无统计学意义(P>0.05),这可能是spvB、spvC基因之间某种基因缺陷可由另一种基因的表达增强来代替,这有待于进一步研究。
| [1] | Nguyen T, Robinson N, Allison SE, et al. IL-10 produced bytrophoblast cells inhibits phagosome maturation leading toprofound intracellular proliferation of Salmonella entericaTyphimurium[J]. Placenta, 2013, 34 (9): 765-74. DOI: 10.1016/j.placenta.2013.06.003. |
| [2] | Su LB, Su CW, Qi YJ, et al. Coinfection with an intestinal helminthimpairs host innate immunity against salmonella enterica serovartyphimurium and exacerbates intestinal inflammation in mice[J]. Infect Immun, 2014, 82 (9): 3855-66. DOI: 10.1128/IAI.02023-14. |
| [3] | Kaur J, Jain SK. Role of antigens and virulence factors ofSalmonella enterica serovar Typhi in its pathogenesis[J]. MicrobiolRes, 2012, 167 (4): 199-210. |
| [4] | Crump JA, Mintz ED. Global trends in typhoid and paratyphoidfever[J]. Clin Infect Dis, 2010, 50 (2): 241-6. DOI: 10.1086/648977. |
| [5] | Wójcik O, KjelsØ C, Kuhn KG, et al. Salmonella typhimuriumoutbreak associated with smoked pork tenderloin in Denmark,January to March 2011[J]. Scand J Infect Dis, 2012, 44 (12): 903-8. DOI: 10.3109/00365548.2012.693196. |
| [6] | Lafuente S, Bellido JB, Moraga FA, et al. Salmonella paratyphi Band Salmonella litchfield outbreaks associated with pet turtleexposure in Spain[J]. Enferm Infecc Microbiol Clin, 2013, 31 (1): 32-5. DOI: 10.1016/j.eimc.2012.05.013. |
| [7] | van Damme P, Kafeja F, Anemona AA, et al. Safety,immunogenicityand dose ranging of a new Vi-CRM197 conjugate vaccineagainst typhoid fever: randomized clinical testing in healthy adults[J]. PLoS One, 2011, 6 (9): e25398. DOI: 10.1371/journal.pone.0025398. |
| [8] | Gole VC, Chousalkar KK, Roberts JR, et al. Effect of egg washingand correlation between eggshell characteristics and egg penetrationby various salmonella typhimurium strains[J]. PLoS One, 2014, 9 (3): e90987. DOI: 10.1371/journal.pone.0090987. |
| [9] | Wei L, Wu SY, Li YY, et al. Salmonella enterica serovar typhiplasmid impairs dendritic cell responses to infection[J]. CurrMicrobiol, 2012, 65 (2): 133-40. |
| [10] | Centers for Disease Control, Prevention. (CDC)[J]. multistate outbreakof human salmonella typhimurium infections associated with petturtleexposure-United states, 2010, 59 (7): 191-6. |
| [11] | Perry JJ, Yousef A. Salmonella enteritidis in shell eggs: evolvingconcerns and innovative control measures[J]. Adv Appl Microbiol, 2012, 81 (5): 243-74. |
| [12] | Kurita A, Gotoh H, Eguchi M, et al. Intracellular expression of theSalmonella plasmid virulence protein,SpvB,causes apoptotic celldeath in eukaryotic cells[J]. Microb Pathog, 2003, 35 (1): 43-8. DOI: 10.1016/S0882-4010(03)00066-4. |
| [13] | Matsui H, Bacot CM, Garlington WA, et al. Virulence plasmidbornespvB and spvC genes can replace the 90-kilobase plasmid inconferring virulence to Salmonella enterica serovar Typhimurium insubcutaneously inoculated mice[J]. J Bacteriol, 2001, 183 (15): 4652-8. DOI: 10.1128/JB.183.15.4652-4658.2001. |
| [14] | Gradmann C. A spirit of scientific rigour: Koch's postulates intwentieth-century medicine[J]. Microbes Infect, 2014, 16 (11): 885-92. DOI: 10.1016/j.micinf.2014.08.012. |
| [15] | Kappeli R, Kaiser P, Stecher B, et al. Roles of spvB and spvC in S[J]. Int J MedMicrobio, 2011, 301 (2): 117-24. |
| [16] | 彭亮, 赵铁, 罗文英, 等. 脑膜炎大肠杆菌K1株ppk1基因致病机制初探[J]. 微生物学通报, 2012, 39 (2): 219-25. |
| [17] | 邱桥成, 叶颖, 储元元, 等. 鼠伤寒沙门菌spvB基因缺陷变异株的构建[J]. 生物技术通报, 2012, 12 : 180-3. |
| [18] | Özkaya H, Akcan AB, Aydemir G, et al. Salmonella typhimuriuminfections in BALB/c mice: a comparison of tissue bioluminescence,tissue cultures and mice clinical scores[J]. NewMicrobiol, 2012, 35 (1): 53-9. |
| [19] | Libby SJ, Lesnick M, Hasegawa P, et al. The salmonella virulenceplasmid spv genes are required for cytopathology in humanmonocyte-derived macrophages[J]. Cell Microbiol, 2000, 2 (1): 49-58. DOI: 10.1046/j.1462-5822.2000.00030.x. |
| [20] | Browne SH, Hasegawa P, Okamoto S, et al. Identification ofsalmonella SPI-2 secretion system components required for SpvBmediatedcytotoxicity in macrophages and virulence in mice[J]. FEMS Immunol Med Microbiol, 2008, 52 (2): 194-201. DOI: 10.1111/j.1574-695X.2007.00364.x. |
| [21] | Su L, Su CW, Qi Y, et al. Coinfection with an intestinal helminthimpairs host innate immunity against Salmonella enterica serovarTyphimurium and exacerbates intestinal inflammation in mice[J]. Infect Immun, 2014, 82 (9): 3855-66. DOI: 10.1128/IAI.02023-14. |
| [22] | Lee KS, Jeong ES, Heo SH, et al. IL-10 suppresses bactericidalresponse of macrophages against Salmonella Typhimurium[J]. JMicrobiol, 2011, 49 (6): 1050-3. |
| [23] | Khader SA, Partida-Sanchez S, Bell GA, et al. Interleukin 12p40 isrequired for dendritic cell migration and T cell priming afterMycobacterium tuberculosis infection[J]. J Exp Med, 2006, 203 (7): 1805-15. DOI: 10.1084/jem.20052545. |
| [24] | Holscher C, Atkinson RA, Arendse B, et al. A protective andagonistic function of IL-12p40 in mycobacterial infection[J]. JImmunol, 2001, 167 (12): 6957-66. DOI: 10.4049/jimmunol.167.12.6957. |
| [25] | Yoon WS, Choi HJ, Park YK. Salmonella typhimurium harboringplasmid expressing interleukin-12 induced attenuation of infectionand protective immune responses[J]. J Gen Appl Microbiol, 2011, 57 (2): 115-22. DOI: 10.2323/jgam.57.115. |
| [26] | Wei L1, Wu S, Li Y, et al. Salmonella enterica serovar Typhiplasmid impairs dendritic cell responses to infection[J]. CurrMicrobiol, 2012, 65 (2): 133-40. |
| [27] | Ehlers S, Lehmann J, Mossmann H, et al. Interleukin-12p40mediates transient protection against Mycobacterium aviuminfection in the absence of interleukin-12[J]. Immunobiology, 2005, 210 (2/4): 217-27. |
| [28] | Pintaric M, Gerner W, Saalmueller A. Synergistic effects of IL-2,IL-12 and IL-18 on cytolytic activity,perforin expression and IFNgammaproduction of porcine natural killer cells[J]. Vet ImmunolImmunopathol, 2008, 121 (1/2): 68-82. |
| [29] | Morselli E, Tasdemir E, Maiuri MC, et al. Mutant p53 proteinlocalized in the cytoplasm inhibits autophagy[J]. Cell Cycle, 2008, 7 (19): 3056-61. DOI: 10.4161/cc.7.19.6751. |
| [30] | Browne SH, Hasegawa P, Okamoto S, et al. Identification of Salmonella SPI-2 secretion system components required for SpvBmediatedcytotoxicity in macrophages and virulence in mice[J]. FEMS Immunol Med Microbiol, 2008, 52 (2): 194-201. DOI: 10.1111/j.1574-695X.2007.00364.x. |
| [31] | Gulig PA, Doyle TJ, Clare-Salzler MJ, et al. Systemic infection ofmice by wild-type but not Spv-Salmonella typhimurium isenhanced by neutralization of gamma interferon and tumor necrosisfactor alpha[J]. Infect Immun, 1997, 65 (12): 5191-7. |
| [32] | Lee KS, Jeong ES, Heo SH, et al. IL-10 suppresses bactericidalresponse of macrophages against Salmonella Typhimurium[J]. JMicrobiol, 2011, 49 (6): 1050-3. |
| [33] | Kobayashi T, Nakatsuka K, Shimizu M, et al. Ribavirin modulatesthe conversion of human CD4(+)CD25(-) T cell to CD4(+)CD25(+)FOXP3(+ ) T cell via suppressing interleukin-10-producingregulatory T cell[J]. Immunology, 2012, 137 (3): 259-70. DOI: 10.1111/imm.12005. |
 2015, Vol. 35
2015, Vol. 35

