2. 中山大学附属第三医院麻醉科,广东 广州 510630
2. Department of Anesthesiology, The Third Affiliated Hospital, Sun Yat-sen University, Guangzhou 510630, Chian
脓毒症,是由于各种病原体感染、创伤、烧伤、缺氧、再灌注损伤及外科大手术后引起的一个严重的、复杂的、失控的、全身性的炎性反应综合症,可引起感染性休克及多器官功能障碍综合征。肾脏是最易受到脓毒症打击的靶器官之一,脓毒症患者中约42%发生急性肾损伤(acute kidney injury,AKI)[1],研究表明约50%的重症AKI患者的病因主要是脓毒症,尽管使用抗生素或免疫调节治疗应用,仍然不能有效地控制病人的多器官功能损伤和病人死亡,由脓毒症引发AKI导致患者死亡率仍然居高不下,脓毒症患者发生AKI 后死亡率高达70%[2-3]。而ICU里需要透析治疗的AKI病人生存率仅有13.8%。因此,为了降低脓毒症导致AKI发病率和改善预后,如何及时预防和有效干预脓毒症并发AKI成为目前一项迫切研究课题。
目前众多研究证实机体脓毒症可导致AKI甚至多器官功能障碍综合征,可能与机体交感神经过度兴奋、免疫失调、炎症反应失控等一系列病理生理改变有关。因此,在脓毒症的治疗中,抑制交感神经过度兴奋和炎症级联放大效应,减轻脓毒症导致AKI是降低脓毒症病死率的有效措施,也是当今危重症医学领域研究的热点和难点。
右美托咪啶(DEX )是选择性α2-肾上腺受体激动剂,对位于脑和脊髓的а2肾上腺素能受体(а2-AR)产生激动效应,产生镇静、镇痛和抑制交感神经等作用,临床上主要用于镇静、镇痛、抑制交感活动等治疗。近年来,越来越多的基础和临床研究证明,DEX能抑制中性粒细胞的激活及TNF-α、IL-1β等炎性细胞因子的释放而发挥全身的抗炎作用,另外也可抑制致炎因子导致的中性粒细胞的呼吸爆发,避免脂质过氧化反应,对心、肾、肺等多器官具有保护作用[4-6]。本研究旨在探讨DEX对脓毒症大鼠AKI模型中肾脏组织细胞的影响,以及炎性因子和氧化应激反应扮演的具体作用机制,希望为AKI的临床治疗提供理论依据。
1 材料与方法 1.1 实验动物和材料雄性清洁级SD大鼠32只,体质量200~250 g(中山大学动物实验中心提供)。右美托咪啶(规格:200 μg/mL, 江苏恒瑞制药厂),育亨宾(yohimbine,YOH)(Sigma-Aldrich),IL-1β测试盒(南京凯基生物试剂有限公司),MDA和SOD测试盒(南京建成生物工程研究所),脂多糖(Sigma-Aldrich)。
1.2 动物分组和模型制备大鼠随机均分为以下4组:Sham组、LPS组、DEX +LPS组和YOH+DEX+LPS组。采用LPS诱导制备脓毒血症肾损伤模型,大鼠常规禁食12 h,不禁饮。诱导麻醉大鼠后,将其放置于变温毯上仰卧,固定,维持体温36±0.1 ℃。除育亨宾经腹腔注射外,DEX和LPS均通过尾静脉注射。具体给药方式如下:LPS组给予LPS 5 mg/kg;DEX +LPS组,首先注射DEX 10 μg/kg,10 min 后同样给予LPS 5 mg/kg;YOH+DEX+LPS组,先经腹腔注射YOH 1 mg/kg,30 min 后注射DEX10 μg/kg,10 min后给予LPS 5 mg/kg。Sham组给予与LPS等量生理盐水注射。
1.3 标本的留取4 h后打开腹腔,经腹主动脉抽取血液,评估IL-1β、SOD及MDA检测。截取左肾脏取部分组织,用10%福尔马林固定,石蜡包埋,切片,行HE染色,光镜下进行组织病理学评估;右肾组织迅速放入液氮中保存,实验结束后转入-80 ℃低温冰箱保存,用于肾组织匀浆检测IL-1β、SOD和MDA水平。
1.4 MDA,SOD和IL-1β检测血和肾组织采用黄嘌呤氧化酶法检测SOD的含量和活性,用硫代巴比妥酶法测定MDA的含量,具体按照操作试剂盒说明书进行,肾组织中的SOD、MDA含量按照公式计算,考马斯亮蓝检测蛋白含量。IL-1β采用酶联免疫分析法(ELISA)操作试剂盒说明书进行。
1.5 统计学分析所得计量数据均以GraphPad Prism 5软件统计,组内比较采用重复测量方差分析,组间比较采用t检验P<0.05 为差异有统计学意义。
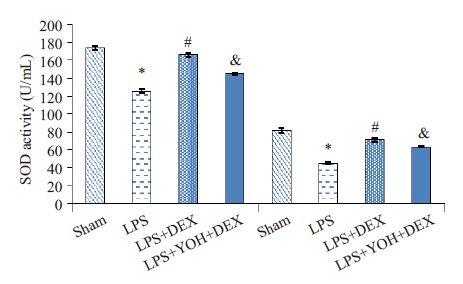
2 结果 2.1 右美托咪啶预处理对血浆和肾组织MDA和SOD的影响 2.1.1 各组血浆和肾组织MDA水平变化与Sham组相比,LPS 组明显增加血浆和肾脏的MDA 含量(P<0.05);与LPS组相比,右美托咪啶预处理组(DEX +LPS组)明显降低血浆和肾脏的MDA含量(P<0.05);与DEX +LPS组相比,α2-肾上腺素受体拮抗剂育亨宾组(YOH+DEX+LPS组)MDA含量上升(P<0.05,图 1)。
|
图 1 各组血浆和肾组织MDA水平的变化 Figure 1 Levels of MDA in the blood and kidney tissue in different groups. Compared with Sham group,LPS group *P<0.05; Compared with LPS group,DEX + LPS group #P<0.05;Compared with DEX+LPS group ,YOH+DEX+LPS group &P<0.05. |
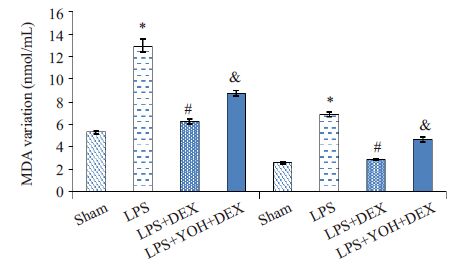
与Sham组相比,LPS组明显降低血浆和肾脏的SOD活力(P<0.05);与LPS组相比,DEX +LPS组明显升高血浆和肾脏的SOD活力(P<0.05);与DEX+LPS组相比,YOH+DEX+LPS组SOD活力下降(P<0.05,图 2)。
|
图 2 各组血浆和肾组织SOD活力的变化 Figure 2 SOD activity in the blood and kidney tissue in different groups. Compared with Sham group,LPS group *P<0.05; Compared with LPS group,DEX+ LPS group #P<0.05; Compared with DEX + LPS group ,YOH + DEX + LPS group &P<0.05. |
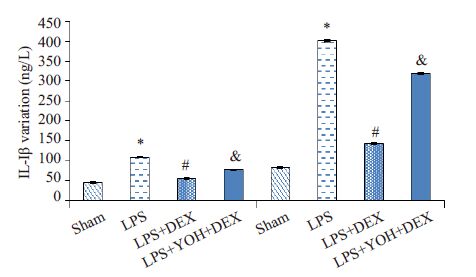
与Sham 组相比,LPS 组明显增加血浆和肾脏的IL-1β含量(P<0.05);与LPS组相比,DEX+LPS组明显降低血浆和肾脏的IL-1β含量(P<0.05);与DEX+LPS组相比,YOH+DEX+LPS组IL-1β含量上升(P<0.05,图 3)。
|
图 3 各组血浆和肾组织IL-1β水平的变化 Figure 3 Levels of IL-1β in the blood and kidney tissue in different groups. Compared with Sham group,LPS group *P<0.05; Compared with LPS group,DEX+ LPS group #P<0.05; Compared with DEX + LPS group, YOH + DEX + LPS group &P<0.05. |
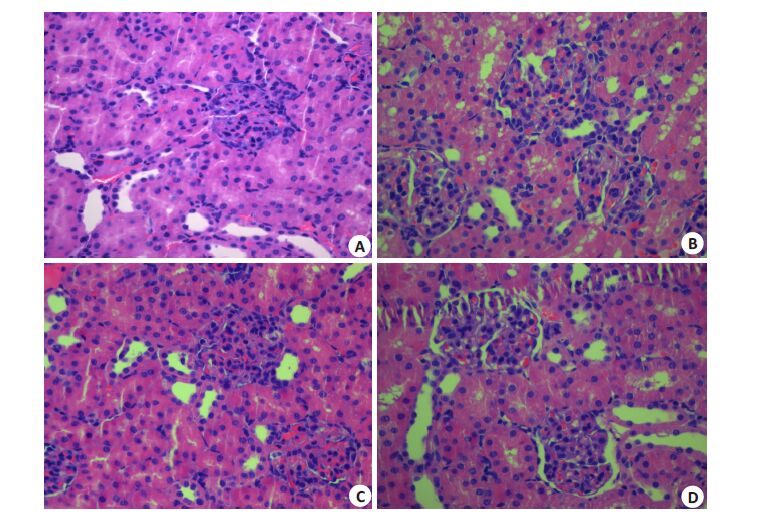
Sham组肾组织结构正常,肾小管、肾间质未见明显的病理性改变;LPS组肾小管上皮细胞肿胀,部分出现空泡变性,肾间质炎症细胞浸润;DEX+LPS组肾脏组织病理损伤减轻,肾小管上皮细胞轻度肿胀或扁平,少量出现空泡样变,肾间质炎症细胞浸润减少;YOH+DEX+LPS组肾脏组织病理损伤较DEX+LPS组加重(图 4)。
|
图 4 各组肾脏组织的病理学改变 Figure 4 Histopathological changes in the kidney tissue in different groups (HE staining, original magnification: × 400). A: SHAM group; B: LPS group; C: DEX+ LPS group; D: YOH+DEX+ LPS group. |
研究发现,DEX预处理大鼠术后4 h,病理学检查发现DEX明显减轻大鼠肾小管上皮细胞变性,肾小管囊腔扩张、空泡变性,肾间质炎症细胞浸润,证明DEX预处理可以显著减轻LPS诱导产生的肾小管损伤。近年来国内外学者研究发现DEX对脑、心、肝、肺、和小肠等多个器官具有保护作用,认为其保护机制是与抗炎、抗氧化损伤与抑制细胞凋亡有关[7-11],本实验通过采用LPS诱导制备大鼠脓毒症AKI模型,应用DEX显著减轻脓毒症大鼠氧化应激和炎症反应,而这一保护作用被DEX特异性受体拮抗剂育亨宾逆转,推测DEX的AKI保护机制可能是通过肾小管上皮细胞上的α2受体起保护作用。
AKI是十分复杂的病理生理过程,其发病机制可能涉及肾缺血再灌注损伤、炎症因子、一氧化氮学说、细胞凋亡学说、内皮功能障碍及内皮素作用、内毒素直接损伤、氧化应激损伤等。LPS相关的AKI与炎症和肾小管的损伤密切相关,α2肾上腺素能受体广泛分布于全身多种器官、组织和细胞,在肾脏,α2受体主要分布于肾近曲小管、集合管及微血管等。DEX是新一代高选择性α2肾上腺素能受体激动剂[12]。DEX具有抗交感兴奋、调节免疫功能、氧化应激及抗炎症反应等作用特点,在脓毒症的治疗及器官保护方面具有显著优势[5, 13-14]。近年来有不少关于DEX对肾脏保护作用机制的研究,认为DEX对脓毒症肾损伤的保护机制主要与抗氧化应激、抗炎症反应及抗细胞凋亡等有关。如Villela等[15]发现DEX可以通过减少肾脏交感神经作用,降低尿渗透压和血浆精氨酸加压素水平,产生利尿作用。Gu等[16]研究指出DEX通过激动α2AR,抑制肾脏细胞凋亡,下调TLR4蛋白表达以及血清HMGB1的水平,是其肾保护的主要机制。另外DEX通过激动α2受体抑制多条信号通路如JAK/STAT信号通路、TLR-4/NF-KB/MAPKs通路下调炎症产物发挥抗炎作用[17-19]。本实验检测了血中的IL-1β水平,发现LPS注射诱导内毒素AKI后IL-1β水平明显增高,DEX可以下调炎症因子IL-1β的表达,该作用被育亨宾部分拮抗。本实验检测结果和国内外学者研究结论是相符的[5, 20-22]。
内毒素血症导致线粒体内呼吸链产生的活性氧簇增加,氧自由基产生增多;另外肾小管上皮细胞内酶活性降低,导致机体产生大量的氧自由基,攻击生物膜中的不饱和脂肪酸,加速脂质过氧化过程,脂质过氧化产物如MDA产生增加,肾小管上皮细胞损伤和功能代谢障碍,甚至产生细胞凋亡,这也是内毒素诱发的肾小管上皮细胞损伤的重要机制之一。我们的结果显示,内毒素血症导致肾组织和血浆中MDA含量明显升高,SOD活力明显下降,表明内毒素注射后机体和肾组织中的氧自由基增加,脂质过氧化程度加剧,抗氧化酶活力下降。DEX预处理能明显降低内毒素血症中肾组织和血浆中的MDA含量,增加SOD活力,提示DEX介导的SOD水平提高可能有助于减少内毒素导致AKI产生的氧自由基,减轻氧化应激,发挥内毒素AKI的保护作用,和国内外的研究一致[23-26],而α2-肾上腺素受体拮抗剂YOH能部分逆转SOD和MDA值的变化。
本实验采用LPS诱导制备大鼠脓毒症模型,这是目前公认的与临床相关性较强的脓毒症模型,证明了DEX可以明显减轻LPS 诱导的急性肾损伤的氧化应激和炎症反应,减轻了肾脏的病理损伤程度,α2-肾上腺素受体拮抗剂YOH能部分拮抗此作用,可以推断DEX对内毒素肾损伤的保护作用可能和免疫炎症调节和抗氧化产物生成密切相关。但其调节信号通路中的具体作用机制尚不清楚,有待于进一步实验探索。
| [1] | Bagshaw SM, George C, Bellomo R, et al. Early acute kidney injury and sepsis: a multicentre evaluation[J]. Crit Care,2008, 12 (2) : R47. DOI: 10.1186/cc6863. |
| [2] | Mehta RL, Bouchard J, Soroko SB, et al. Sepsis as a cause and consequence of acute kidney injury: program to improve care in acute renal disease[J]. Intensive Care Med,2011, 37 (2) : 241-8. DOI: 10.1007/s00134-010-2089-9. |
| [3] | Rajapakse S, Rodrigo C, Rajapakse A, et al. Renal replacement therapy in sepsis induced acute renal failure[J]. Saudi J Kidney Dis Transpl,2009, 20 (4) : 553-9. |
| [4] | Sanders RD, Sun P, Patel S, et al. Dexmedetomidine provides cortical neuroprotection: impact on anaesthetic-induced neuroapoptosis in the rat developing brain[J]. Acta Anaesthesiol Scand,2010, 54 (6) : 710-6. |
| [5] | Taniguchi T, Kurita A, Kobayashi K, et al. Dose-and time-related effects of dexmedetomidine on mortality and inflammatory responses to endotoxin-induced shock in rats[J]. J Anesth,2008, 22 (3) : 221-8. DOI: 10.1007/s00540-008-0611-9. |
| [6] | Hofer S, Steppan J, Wagner T, et al. Central sympatholytics prolong survival in experimental sepsis[J]. Crit Care, 2009, 13(1): R11.[J]. Crit Care,2009, 13 (1) : R11. DOI: 10.1001/jama.2009.56. |
| [7] | Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial[J]. JAMA,2009, 301 (5) : 489-99. |
| [8] | Mantz J, Josserand J, Hamada S. Dexmedetomidine: new insights[J]. Eur J Anaesthesiol,2011, 28 (1) : 3-6. DOI: 10.1097/EJA.0b013e32833e266d. |
| [9] | Hanci V, Yurdakan G, Yurtlu S, et al. Protective effect of dexmedetomidine in a rat model of alpha-naphthylthioureaeinduced acute lung injury[J]. J Surg Res,2012, 178 (1) : 424-30. DOI: 10.1016/j.jss.2012.02.027. |
| [10] | Gu J, Chen J, Xia P, et al. Dexmedetomidine attenuates remote lung injury induced by renal ischemia-reperfusion in mice[J]. Acta Anaesthesiol Scand,2011, 55 (10) : 1272-8. DOI: 10.1111/aas.2011.55.issue-10. |
| [11] | Gazit AZ. Dexmedetomidine and pulmonary artery pressure in the pediatric cardiac surgery patient-A new insight[J]. Pediat Cri Care Med,2010, 11 (5) : 634-5. DOI: 10.1097/PCC.0b013e3181d505e3. |
| [12] | Kaur M, Singh PM. Current role of dexmedetomidine in clinical anesthesia and intensive care[J]. Anesth Essays and Res,2011, 5 (2) : 128-33. DOI: 10.4103/0259-1162.94750. |
| [13] | Hardin KA. Sleep in the ICU potential mechanisms and clinical implications[J]. Chest,2009, 136 (1) : 284-94. DOI: 10.1378/chest.08-1546. |
| [14] | Lin Y, Zhu X, Yao WZ, et al. Yohimbine protects against endotoxin-induced acute lung injury by blockade of alpha 2A adrenergic receptor in rats[J]. Chin Med J (Engl),2011, 124 (7) : 1069-74. |
| [15] | Villela NR, Do Nascimento JP, Carvalho LR, et al. Effects of dexmedetomidine on renal system and on vasopressin plasma levels[J]. Rev Bras Anesthesiol,2005, 55 (4) : 429-40. |
| [16] | Gu J, Chen J, Xia P, et al. Dexmedetomidine attenuates remote lung injury induced by renal ischemia-reperfusion in mice[J]. Acta Anaesthesiol Scand,2011, 55 (10) : 1272-8. DOI: 10.1111/aas.2011.55.issue-10. |
| [17] | Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987-2007) J][J]. Brain Behav Immun,2007, 21 (6) : 736-45. DOI: 10.1016/j.bbi.2007.03.008. |
| [18] | Si Y, Bao H, Han L, et al. Dexmedetomidine protects against renal ischemia and reperfusion injury by inhibiting the JAK/STAT signaling activation[J]. J Transl Med,2013, 11 (1) : 141. DOI: 10.1186/1479-5876-11-141. |
| [19] | Lai YC, Tsai PS, Huang CJ. Effects of dexmedetomidine on regulating endotoxin-induced up-regulation of inflammatory molecules in murine macrophages[J]. J Surg Res,2009, 154 (2) : 212-9. DOI: 10.1016/j.jss.2008.07.010. |
| [20] | Hsing CH, Lin CF, So E, et al. α2-A adrenoceptor agonist demedetomidine protects septic acute kidney injury through increasing BMP-7 and inhibiting HDAC2 and HDAC5[J]. Am J Physiol Renal Physiol,2012, 303 (10) : F1443-53. DOI: 10.1152/ajprenal.00143.2012. |
| [21] | Yang CL, Tsai PS, Huang CJ. Effects of dexmedctomidine on regulating pulmonary inflammation in a rat model of ventilatorinduced lung injury[J]. Acta Anaesthesiol Taiwan,2008, 46 (4) : 151-9. DOI: 10.1016/S1875-4597(09)60002-3. |
| [22] | Tasdogan M, Memis D, Sut N, et al. Results of a pilot study on the effeetsof propofol and dexmedetomidine on inflammatory responses and intraabdominal pressure in severe sepsis[J]. J Clin Anesth,2009, 21 (6) : 394-400. DOI: 10.1016/j.jclinane.2008.10.010. |
| [23] | Eser O, Fidan H, Sahin O, et al. The influence of dexmedetomidine on ischemic rat hippocampus[J]. Brain Res,2008, 1218 (7) : 250-6. |
| [24] | Yoshitomi O, Cho S, Hara T, et al. Direct protective effects of dexmedetomidine against myocardial ischemia-reperfusion injury in anesthetized pigs[J]. Shock,2012, 38 (1) : 92-7. DOI: 10.1097/SHK.0b013e318254d3fb. |
| [25] | Sahin T, Begeç Z, Toprak Hİ, et al. The effects of dexmedetomidine on liver ischemia-reperfusion injury in rats[J]. J Surg Res,2013, 183 (1) : 385-90. DOI: 10.1016/j.jss.2012.11.034. |
| [25] | Zhang XY, Liu ZM, Wen SH, et al. Dexmedetomidine administration before, but not after, ischemia attenuates intestinal injury induced by intestinal ischemia-reperfusion in rats[J]. Anesthesiology,2012, 116 (5) : 1035-46. DOI: 10.1097/ALN.0b013e3182503964. |
 2015, Vol. 35
2015, Vol. 35

