Ganoderma lucidum is commonly used for prevention and treatment of a variety of diseases including immunological disorders [1], infections [2, 3], chronic hepatitis, gastric ulcer[4], hypoglycemic[5, 6], and scleroderma tumorigenic diseases[7-11]. Ganoderic acids (GAs), identified as triterpenoids, are the major bioactive compounds found in G. lucidum. Among these GAs, GA-A was demonstrated to control lymphoma growth both in vitro and in vivo[12] and enhance the chemosensitivity of HepG2 cells to cisplatin by inhibiting the JAK-STAT3 signaling pathway[13]. GA-A and GA-H were found to suppress the growth and invasiveness of breast cancer cells by inhibiting the transcription factors AP-1 and nuclear factor-κB (NF-κB) [14].
Osteosarcoma is the most commonly diagnosed primary malignant tumor of the bone in human. Approximately 60% of osteosarcoma occur in patients under 20 years of age[15-17], and its treatment remains a challenge. It has been demonstrated that SCGLP1, a sulfated polysaccharide from the fruiting bodies of G. lucidum, can produce significant inhibitory effect on MG63 cell proliferation and viability and cause cell apoptosis with only minor cytotoxic effect on normal human osteoblasts [18]. So far few studies have been reported to examine the bioactivity of purified triterpenes against osteosarcoma cells. In this study, we aimed to examine the effect of GA-A on osteosarcoma cell proliferation, apoptosis and invasion and explore the possible mechanisms underlying these effects by examining 3 important signaling pathways.
MATERIALS AND METHODS Cell cultureHuman osteosarcoma HOS and MG-63 cells were supplied by the Institute of Cell Bank/Institutes for Biological Sciences (Shanghai, China). HOS cells were cultured in minimum essential medium (MEM) supplemented with 10% fetal bovine serum (FBS). MG63 cells were maintained in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% FBS. These cells were cultured in a humidified incubator with 95% air and 5% CO2 at 37 ℃.
Cell treatmentPurified GA-A (purity >96% by HPLC), obtained from Chromadex, was dissolved in methanol at the concentration of 50 mmol/L and stored at 4 ℃. The cells were exposed to 0.10, 0.25, and 0.50 mmol/L GA-A[14] and the changes in their biological behaviors were evaluated, using cells treated with 0.2 ml 1% methanol as control.
Cell proliferation assayThe cells (5 × 103/ml) incubated in a 96-well plate were treated with 0.2 ml ethanol solution of GA-A of indicated concentrations for 24, 48 or 72 h at 37 ℃. The change of cell proliferation was evaluated using MTT assay kit (Promega) following the manufacturer's protocol. Briefly, 10 μl of MTT reagent was added to each well and the plate was incubated for 4 h at 37 ℃. After the medium was removed, 200 μl of dimethyl sulfoxide was added to each well to dissolve formazan formed. After 30 min of incubation at room temperature, the absorbance was measured at 570 nm using a Vmax microplate spectrophotometer (Molecular Devices, Sunnyvale, CA, USA). The IC50 value was calculated by nonlinear regression analysis with GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA), using the dose-response with variable slope function. Each sample was assayed in triplicate.
Cell apoptosis assayHOS and MG-63 cells were harvested 48 h after GA-A treatment and washed twice with PBS after centrifugation at 2000 r/min for 5 min. The cells (5×105) were resuspended with 500 μl binding buffer from a KGA107 kit (KeyGene, Nanjing, China) followed by the addition of 5 μl of Annexin V-fluorescein isothiocyanate (FITC) and then 5 μl of propidium iodide. After incubation for 10 min at room temperature in darkness, the cells were analyzed immediately by flow cytometry.
Cell invasion assayHOS and MG-63 cells treated with GA-A for 24 h were transferred to the top compartment of Matrigel-coated chambers (24-well insert, 8-μm pore size, BD Biosciences, San Jose, USA) which contained serum-free DMEM or MEM. The medium containing 20% FBS was added to the lower chamber as a chemoattractant. After incubation for 24 h, the cells that did not invade were removed from the upper chamber with cotton swabs while the invasive cells were fixed with 4% paraformaldehyde, stained with hematoxylin, and photographed (×200) in five random fields for each well. The number of invasive cells was counted using Image-Pro Plus software and averaged from the 5 fields. Each assay was repeated for 3 times.
Western blottingTo investigate the possible molecular mechanism underlying the inhibitory effect of GA-A on HOS and MG-63 cells, we detected the protein expression levels of STAT3, p-STAT3, p38, p-p38 and NF-κB1 with Western blotting. The primary antibodies and secondary antibodies were purchased from CST (Boston, USA),
HOS and MG-63 cells (2×106) were collected and washed twice with ice-cold PBS. The cell pellets were suspended in RIPA lysis buffer for 40 min on ice, and the lysates were centrifuged at 12 000 g at 4 ℃ for 15 min. Equal amounts of proteins in the supernatant were loaded for 10% SDS polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto PVDF membrane (PALL, USA). The membranes were blocked with 5% skim milk for 90 min at 37 ℃ and then incubated with the primary antibodies at the optimal dilutions (1: 1000) in 5% skim milk for 1 h at 37 ℃. After washing with TBS containing 0.5% Tween 20 (TBST), the membrane was incubated with HRP-conjugated secondary antibody (1: 5000) at room temperature for 40 min. After further washing with TBST, the membrane was developed with enhanced chemiluminescence (ECL) and visualized on X-ray films. The intensities of the protein bands were quantified using Image-Pro Plus software. NF-κB1 blot values were corrected for GAPDH expression, and the blot values for phosphorylated proteins were corrected for total protein and GAPDH expression.
Statistical analysisStatistical analysis was performed using SPSS 13.0 software package (SPSS Inc, Chicago, IL, USA). The data of cell proliferation was analyzed using One-way ANOVA analysis. The cell apoptosis rate was compared using Chi-square test between the groups. The other numerical data was analyzed using Student's t-test. All tests performed were two-sided. A P value less than 0.05 was considered to indicate a statistically significant difference.
RESULTS Effect of GA-A on HOS and MG-63 cell proliferationAs shown in Fig. 1A, 0.1 mmol/L GA-A caused no obvious inhibition of HOS cell growth compared with the control group, while 0.25 and 0.5 mmol/L GA-A significantly inhibited the growth of HOS cells at 48 h and 72 h, but not at 24 h. Similar dose-and time-related inhibitory effects of GA-A were also observed in MG-63 cells (Fig. 1B). The IC50 of GA-A for HOS cells at 24, 48 and 72 h was 0.81, 0.51, and 0.48 mmol/L, and that for MG-63 cells was 0.80, 0.50 and 0.47 mmo/L, respectively. Based on these results, we chose 0.5 mmol/L GA-A for 48 h as the optimal cell treatment condition in the following assays.
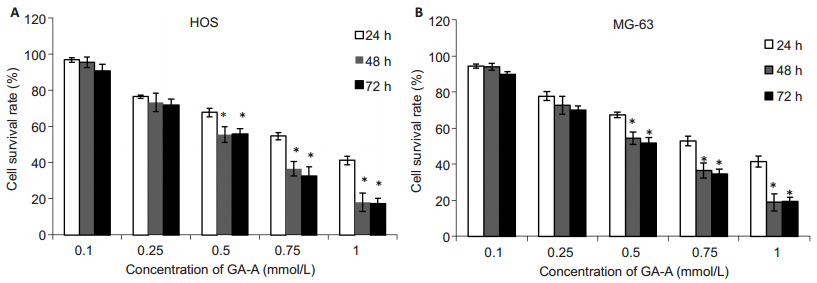
|
Figure 1 Effect of different concentrations of GA-A on the proliferation of HOS (A) and MG-63 (B) cells determined by MTT assay. Data are Mean±SD from triplicate determinations. *P < 0.05 vs 24 h. |
Flow cytometric analysis showed that treatment with 0.5 mmol/L GA-A for 48 h significantly increased early cell apoptosis rate as compared with the control samples (Fig. 2). In the control and GA-A treatment groups, the early cell apoptosis rates were (5.4±1.2)% and (16.2±2.8)% in HOS cells, and were (4.6±1.4)% and (17.6±2.1)% in MG-63 cells, respectively.
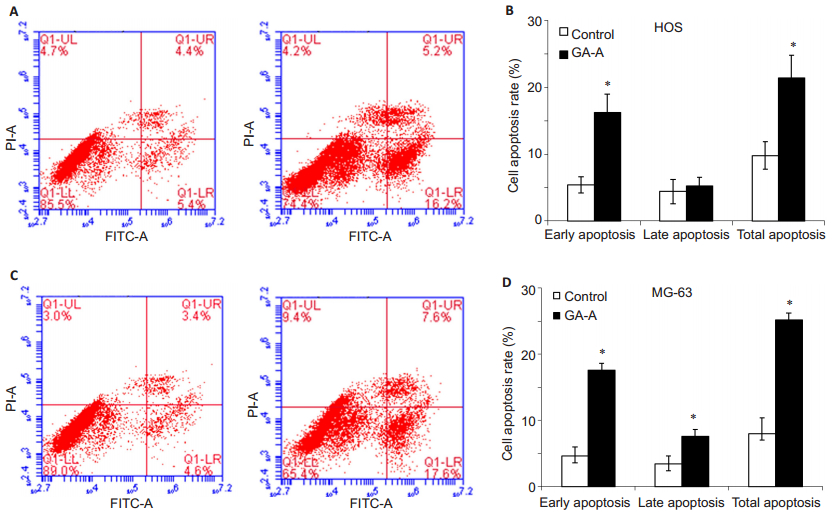
|
Figure 2 Flow cytometry of apoptosis of HOS and MG-63 cells induced by 0.5 mmol/L GA-A for 48 h. A: Flow cytometry analysis of HOS cells; B: Comparison of early, late and total apoptosis rates of HOS cells between the control and treatment groups; C: Flow cytometry analysis of MG-63 cells; D: Comparison of early, late and total apoptosis rates of MG-63 cells between the two groups. The data are presented as Mean±SD. *P < 0.05 vs control. |
As shown in Fig. 3, the number of HOS cells that passed through Matrigel-coated membrane to the lower chamber was significantly lower in GA-A treatment group than in the control group (81±28 vs 233±41, P < 0.05). Similarly, GA-A treatment also significantly lowered the invasive cell number of HOS cells compared with the control cells (150±18 vs 339±23, P < 0.05).
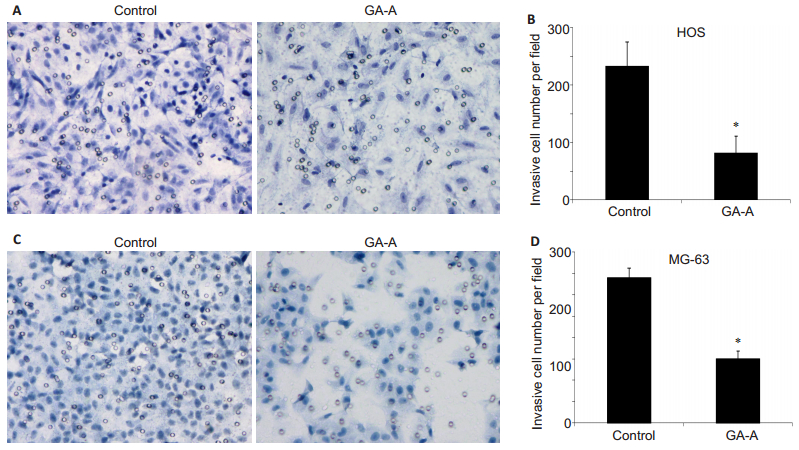
|
Figure 3 Effect of GA-A treatment on cell invasion. A: Invasive HOS cells in GA-A and control groups; B: Quantitative analysis of invasive HOS cells per field in the two groups. C: Invasive MG-63 cells in GA-A and control groups; D: Quantitative analysis of invasive cell number for MG-63 cells. Data are presented as Mean±SD. *P < 0.05. |
As shown in Fig. 4, GA-A treatment significantly decreased p-STAT3 expression levels in both HOS and MG-63 cells by 38.5% and 58.8%, respectively. The expression of phosphorylated p38 (p-p38) and p38 were increased after GA-A treatment by 4.85 folds in HOS cells and by 3.18 folds in MG-63 cells. GA-A treatment also resulted in significant increases in the expression level of NF-κB1 in HOS and MG-63 cells by 3.77 and 3.16 folds, respectively.
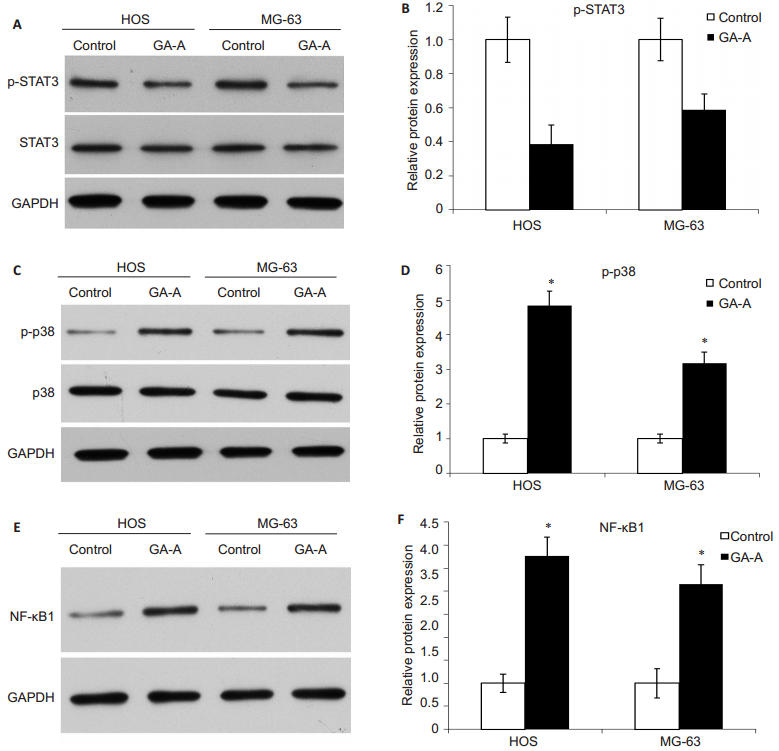
|
Figure 4 Protein expressions of STAT3, p-p38, and NF-κB1 in HOS and MG-63 cells after treatment with GA-A. A, B: Western blotting for STAT3 and quantification of its expression level relative to the control level; C, D: Western blotting for p-p38 and p38 and quantification of the results (presented as fold increase relative to the control); E, F: Western blotting for NF-κB1 and quantification of the results (presented as fold increase). *P < 0.05 GA-A vs control. |
We demonstrated in this study that GA-A could inhibit cell proliferation, induce apoptosis and suppress invasion of human osteosarcoma cells in vitro. So far the effects of GA-A were tested only in colon cancer, gastric cancer, hepatocellular carcinoma and breast cancer cells [12, 19]. In breast cancer MDA-MB-231 cells, GA-A was found to suppress the cell proliferation, colony formation, and invasive behaviors [13], similar to our findings in the two osteosarcoma cell lines. We explored the possible molecular mechanisms underlying the inhibitory effects of GA-A and found that GA-A treatment of the cells caused up-regulated expressions of p-p38 and NF-κB1 but down-regulated the expression level of p-STAT3 in both of the two cell lines.
Compelling evidence supports the critical role of STAT3 activation in regulating tumor cell metastasis, proliferation, apoptosis, invasion, migration and angiogenesis [20]. Persistent activation of STAT3 is detected in a wide variety of human cancer cell lines and tumor tissues [21, 22]. Several previous studies demonstrated that inhibition of STAT3 with a dominant negative form of STAT3 or other inhibitors suppressed the proliferation and survival of various cancers [23]. According to our results, GA-A can obviously inhibit STAT3 activation by decreasing its phosphorylation level in osteosarcoma cells. In human hepatic carcinoma HepG2 cells, inhibition of the JAK-STAT3 signaling pathway by GA-A enhances the chemosensitivity of cells to cisplatin[13]. These evidences, taken together, revealed that GA-A is a potential inhibitor of STAT3.
Mitogen-activated protein kinases (MAPKs) mediate various cellular behaviors in response to extracellular stimuli. The p38 MAP kinases, one of its main subgroups, have been implicated in a wide range of complex biological processes of cancer cells, such as cell proliferation, differentiation, cell death, cell migration, and invasion [24]. Liang et al [25] examined relationship between gallic acid and p38 signaling pathway in human osteosarcoma cells both in vitro and in vivo, and their findings demonstrated that gallic acid reduced osteosarcoma cell viability by inducing apoptosis as a result of activation of the p38 signal pathway. Similar to these results, we found that GA-A could activate the p38 signal pathway, which is involved in the activation NF-κB[26]. We also found that NF-κB1, a member of NF-κB family, was activated after GA-A treatment. These findings indicate that GA-A suppress osteosarcoma cell proliferation and invasion and induce cell apoptosis possibly by activating the p38-NF-κB signal pathway.
In conclusion, we demonstrated that GA-A could inhibit the growth and invasive behaviors and induced cell apoptosis of human osteosarcoma cells. GA-A can simultaneously inactivate STAT3 and activate p38-NF-κB signal pathway, and these signaling pathways merit further investigation for their potential roles in mediating the effects of GA-A on human osteosarcoma cells.
| [1] | Huang SQ, Ning ZX. Extraction of polysaccharide from Ganoderma lucidum and its immune enhancement activity[J]. Int J Biol Macromol,2010, 47 (3) : 336-41. DOI: 10.1016/j.ijbiomac.2010.03.019. |
| [2] | Liu DZ, Zhu YQ, Li XF, et al. New triterpenoids from the fruiting bodies of Ganoderma lucidum and their bioactivities[J]. Chem Biodivers,2014, 11 (6) : 982-6. DOI: 10.1002/cbdv.201400004. |
| [3] | Vazirian M, Faramarzi MA, Ebrahimi SE, et al. Antimicrobial effect of the Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (higher Basidiomycetes) and its main compounds[J]. Int J Med Mushrooms,2014, 16 (1) : 77-84. DOI: 10.1615/IntJMedMushr.v16.i1. |
| [4] | Batra P, Sharma AK, Khajuria R. Probing Lingzhi or Reishi medicinal mushroom Ganoderma lucidum (higher Basidiomycetes): a bitter mushroom with amazing health benefits[J]. Int J Med Mushrooms,2013, 15 (2) : 127-43. DOI: 10.1615/IntJMedMushr.v15.i2. |
| [5] | Li F, Zhang Y, Zhong Z. Antihyperglycemic effect of Ganoderma lucidu polysaccharides on streptozotocin-induced diabetic mice[J]. Int J Mol Sci,2011, 12 (9) : 6135-45. |
| [6] | Xiao C, Wu QP, Cai W, et al. Hypoglycemic effects of Ganoderma lucidum polysaccharides in type 2 diabetic mice[J]. Arch Pharm Res,2012, 35 (10) : 1793-801. DOI: 10.1007/s12272-012-1012-z. |
| [7] | Xu Z, Chen X, Zhong Z, et al. Ganoderma lucidum polysaccharides: Immunomodulation and potential anti-tumor activities[J]. Am J Chin Med,2011, 39 (1) : 15-27. DOI: 10.1142/S0192415X11008610. |
| [8] | Liang Z, Yi Y, Guo Y, et al. Chemical characterization and antitumor activities of polysaccharide extracted from Ganoderma lucidum[J]. Int J Mol Sci,2014, 15 (5) : 9103-16. DOI: 10.3390/ijms15059103. |
| [9] | Loganathan J, Jiang J, Smith A, et al. The mushroom Ganoderma lucidum suppresses breast-to-lung cancer metastasis through the inhibition of pro-invasive genes[J]. Int J Oncol,2014, 44 (6) : 2009-15. |
| [10] | Oliveira M, Reis FS, Sousa D, et al. A methanolic extract of Ganoderma lucidum fruiting body inhibits the growth of a gastric cancer cell line and affects cellular autophagy and cell cycle[J]. Food Funct,2014, 5 (7) : 1389-94. DOI: 10.1039/c4fo00258j. |
| [11] | Shen J, Park HS, Xia YM, et al. The polysaccharides from fermented Ganoderma lucidum mycelia induced miRNAs regulation in suppressed HepG2 cells[J]. Carbohydr Polym,2014, 103 : 319-24. DOI: 10.1016/j.carbpol.2013.12.044. |
| [12] | Radwan FF, Hossain A, God JM, et al. Reduction of myeloid-derived suppressor cells and lymphoma growth by a natural triterpenoid[J]. J Cell Biochem,2015, 116 (1) : 102-14. DOI: 10.1002/jcb.v116.1. |
| [13] | Yao X, Li G, Xu H, et al. Inhibition of the JAK-STAT3 signaling pathway by ganoderic acid a enhances chemosensitivity of HepG2 cells to cisplatin[J]. Planta Med,2012, 78 (16) : 1740-8. DOI: 10.1055/s-00000058. |
| [14] | Jiang J, Grieb B, Thyagarajan A, et al. Ganoderic acids suppress growth and invasive behavior of breast cancer cells by modulating AP-1 and NF-kappaB signaling[J]. Int J Mol Med,2008, 21 (5) : 577-84. |
| [15] | Smeland S, Muller C, Alvegard TA, et al. Scandinavian sarcoma group osteosarcoma study SSG Ⅷ: prognostic factors for outcome and the role of replacement salvage chemotherapy for poor histological responders[J]. Eur J Cancer,2003, 39 (4) : 488-94. DOI: 10.1016/S0959-8049(02)00747-5. |
| [16] | Ferrari S, Smeland S, Mercuri M, et al. Neoadjuvant chemotherapy with high-dose Ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups[J]. J Clin Oncol,2005, 23 (34) : 8845-52. DOI: 10.1200/JCO.2004.00.5785. |
| [17] | Mialou V, Philip T, Kalifa C, et al. Metastatic osteosarcoma at diagnosis: prognostic factors and long-term outcome-the French pediatric experience[J]. Cancer,2005, 104 (5) : 1100-9. DOI: 10.1002/(ISSN)1097-0142. |
| [18] | Sun Z, Huang K, Fu X, et al. A chemically sulfated polysaccharide derived from Ganoderma lucidum induces mitochondrial-mediated apoptosis in human osteosarcoma MG63 cells[J]. Tumour Biol,2014, 35 (10) : 9919-26. DOI: 10.1007/s13277-014-2217-1. |
| [19] | Liang Z, Guo YT, Yi YJ, et al. Ganoderma lucidum polysaccharides target a Fas/caspase dependent pathway to induce apoptosis in human colon cancer cells[J]. Asian Pac J Cancer Prev,2014, 15 (9) : 3981-6. DOI: 10.7314/APJCP.2014.15.9.3981. |
| [20] | Kamran MZ, Patil P, Gude RP. Role of STAT3 in cancer metastasis and translational advances[J]. Biomed Res Int,2013, 2013 : 421821. |
| [21] | Bowman T, Garcia R, Turkson J, et al. STATs in oncogenesis[J]. Oncogene,2000, 19 (21) : 2474-88. DOI: 10.1038/sj.onc.1203527. |
| [22] | Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention[J]. Clini Cancer Res,2002, 8 (4) : 945-54. |
| [23] | Niu G, Heller R, Catlett-Falcone R, et al. Gene therapy with dominant-negative Stat3 suppresses growth of the murine melanoma B16 tumor in vivo[J]. Cancer Res,1999, 59 (20) : 5059-63. |
| [24] | Koul HK, Pal M, Koul S. Role of p38 MAP Kinase Signal Transduction in Solid Tumors[J]. Genes Cancer,2013, 4 (9-10) : 342-59. DOI: 10.1177/1947601913507951. |
| [25] | Liang CZ, Zhang X, Li H, et al. Gallic acid induces the apoptosis of human osteosarcoma cells in vitro and in vivo via the regulation of mitogen-activated protein kinase pathways[J]. Cancer Biother Radiopharm,2012, 27 (10) : 701-10. DOI: 10.1089/cbr.2012.1245. |
| [26] | Schulze-Osthoff K, Ferrari D, Riehemann K, et al. Regulation of NF-kappa B activation by MAP kinase cascades[J]. Immunobiology,1997, 198 (1-3) : 35-49. DOI: 10.1016/S0171-2985(97)80025-3. |
 2015, Vol. 35
2015, Vol. 35
