2. 新乡医学院基础医学院机能学实验室,河南 新乡 453003
2. Department of Functional Laboratory, College of Basic Medical Science, Xinxiang Medical University, Xinxiang 453003, China
呼吸是生命存在的前提和基本条件,呼吸运动是呼吸肌在呼吸中枢支配下节律性舒缩完成的。延髓是呼吸放电的产生部位,在本实验中所使用的新生大鼠延髓脑片包括面神经后核内侧区(the medial region of the nucleus retrofacialis, mNRF),而该区域是延髓内基本节律性呼吸放电产生和调节的精确部位[1-2]。
女性孕期摄入酒精抑制胎儿各系统、器官的发育,尤其对中枢神经系统发育的影响更严重[3]。有研究表明,孕期母体饮酒能够降低幼鼠的每分通气量,损伤呼吸系统网络在低氧情况下的反应性[4]。但孕期摄入酒精对新生动物呼吸中枢作用的报导尚不多见:Duncan等研究发现孕期饮酒降低了婴儿猝死综合征死亡的婴儿脑干呼吸循环相关核团神经元(3)H-nicotinic受体浓度,并随着饮酒量的增加呈剂量依赖性降低[5];Conradt等发现孕期饮酒增加1月龄婴儿的呼吸性窦性心律不齐发生几率[6]。而孕期摄入酒精对新生动物延髓呼吸中枢基本节律性呼吸放电的作用未见报导。为此,我们通过记录分析孕期酒精暴露对新生大鼠延髓脑片基本节律性呼吸放电(RRDA)来研究孕期酒精暴露对新生大鼠延髓呼吸中枢的作用。
1 材料与方法 1.1 实验动物和分组30只SPF级Sprague-Dawley成年大鼠,雌雄比例2:1,购自郑州大学实验动物中心(许可证号SCXK豫2010-002),雌鼠和雄鼠随机分为数量相等的5组对照组、4%酒精暴露组、6%酒精暴露组、8%酒精暴露组和10%酒精暴露组(酒精浓度为体积/体积),按照雌雄大鼠2:1合笼交配。对照组大鼠自由饮用纯净水,不同浓度酒精暴露组大鼠自合笼前1周至产后3 d以相应浓度酒精水溶液作为唯一饮用水(自由饮用)。实验使用各组2 d龄新生大鼠(每组6只),雌雄不拘。
1.2 试剂无水乙醇,天津市德恩化学试剂有限公司生产;改良Kreb's液(modified Kreb's solution, MKS)in mmol/L:NaCl 124、KCl 5、CaCl2 2.4、MgSO4 1.3、NaHCO3 26、Glucose 30所用药品购自Sigma;95% O2+5% CO2混合气购自河南源正科技发展有限公司。
1.3 脑片制备及电生理记录2 d龄5组新生大鼠各6只被乙醚深度麻醉后在C4~C5之间断头,将鼠头置于盛有95% O2+5% CO2混合气充分饱和的冰MKS的解剖盒内。眼科剪剪去皮肤和肌肉,从颈椎处剪开椎管并向吻端延伸,完整剪开颅骨、去除颅骨、暴露脑组织,手术刀片割断颅底脑神经、游离出完整的脑组织。夹去小脑、在C2~C3间切去脊髓,将脑组织腹侧面向上,在闩前后切下厚约900~1100 μm的延髓脑片,在操作过程中持续通95% O2+5% CO2的混合气。脑片切好后转移至脑片灌流槽内,使用混合气充分饱和并持续通气的MKS以5~7 ml/min的速度灌流,保持灌流液pH7.35~7.45、温度25~28 ℃ [7-8]。在体视显微镜下辨认脑片腹侧面舌下神经根并用内含银-氯化银电极的吸附电极(内径180~220 μm)在舌下神经根处加以负压吸附,记录RRDA。RRDA经直流前置放大器放大后输入BL-420F生物信号采集和处理系统在计算机显示器上显示并存盘,以备实验后数据统计分析[8]。
1.4 数据处理为直观简洁对比实验结果,分别以对照组RRDA的吸气时程(inspiratory time, TI)、呼吸频率(respiratory frequency, RF)、放电积分幅度(integral amplitude, IA)等观测指标为100%,对各组10、20、30、40、50 min时间点RRDA进行标准化处理后再进行统计分析。计算公式:各浓度组原始数据/对照组原始数据×100%。
1.5 统计学方法实验结果以均数±标准差的形式表示,使用SPSS 13.0统计软件重复测量方差分析和单因素方差分析检验,多重比较采用LSD法进行分析,P < 0.05被认为有显著性差异。
2 结果 2.1 对照组RRDA经统计学分析,与10 min相比20、30、40、50 min时间点RRDA的TI、IA、RF无统计学差异,说明RRDA在实验过程中无衰减,证明本实验模型稳定可靠(P>0.05)(表 1,图 1)
| 表 1 对照组不同时间点RRDA Table 1 RRDA in the control group at different time points |

|
图 1 对照组在50 min内不同时间点RRDA Figure 1 RRDA in 50 min in the control group. |
4%酒精暴露组、6%酒精暴露组、8%酒精暴露组各组内新生大鼠脑片RRDA各指标在不同时间点无统计学差异(P>0.05,表 2~4)成年大鼠饮用水中酒精浓度增加,新生鼠RRDA逐渐降低,TI缩短、RF下降、IA减弱。10%酒精暴露组RRDA节律不规整,并且弱于8%酒精暴露组。因此,我们选择8%酒精作为孕期酒精暴露作用的最适浓度(图 2)。
| 表 2 4%酒精暴露组不同时间点RRDA Table 2 RRDA in 4% alcohol exposure group at different time points |
| 表 3 6%酒精暴露组不同时间点RRDA Table 3 RRDA in 6% alcohol exposure group at different time points |
| 表 4 8%酒精暴露组不同时间点RRDA Table 4 RRDA in 8% alcohol exposure group at different time points |
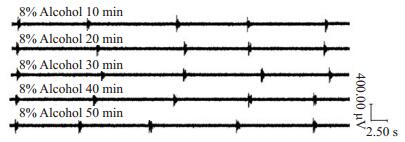
|
图 2 8%酒精暴露组在50 min内不同时间RRDA Figure 2 RRDA in 8% alcohol exposure group at different time points in 50 min. |
孕期酒精暴露抑制剂量依赖性的新生大鼠延髓脑片RRDA,浓度为4%的孕期酒精暴露对RRDA的抑制作用已有统计学差异,RF低于对照组,6%酒精暴露组TI、RF、IA低于对照组和4%酒精暴露组;8%酒精暴露组TI、RF、IA均低于对照组、4%酒精暴露组和6%酒精暴露组(图 3)。与对照组相比,孕期酒精暴露组新生大鼠延髓脑片RRDA的TI缩短,RF下降,IA减弱,具有统计学差异(P < 0.05,表 5,图 4)。
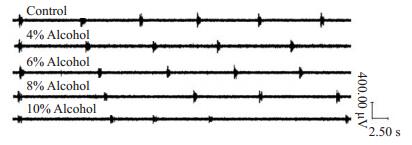
|
图 3 对照组和不同浓度酒精暴露组RRDA的比较 Figure 3 Comparison of RRDA in neonatal rats among the groups. Prenatal exposure to increased alcohol concentrations attenuated RRDA in the medullary slices of the neonatal rats with shortened TI and decreased IA and RF. |
| 表 5 各组30 min时RRDA数据比较 Table 5 Comparison of RRDA among the groups at 30 min |
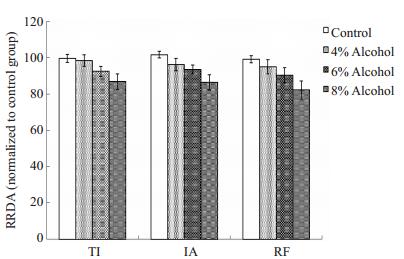
|
图 4 不同浓度酒精暴露对新生大鼠RRDA的影响 Figure 4 Influence of prenatal exposure to different alcohol concentrations on RRDA of neonatal rats. RRDA in the alcohol exposure groups were lower than those in the control group. |
近年来,关于酒精暴露对神经系统的影响已成为国内外研究的热点。在神经系统发育早期,即神经元突触形成时期,短暂接触酒精就能引起神经元的大量死亡。慢性酒精暴露能够影响神经元的分化和迁移,影响突触和轴突的生成,并可通过影响Na+/K+-ATP酶、腺苷酸环化酶的活性以及Ca2+通道开放,诱导神经元凋亡[9],引起形态结构发育障碍,进而引起胎儿发育不良。而婴儿发育不良的程度与母亲孕期的饮酒量、饮酒时间和次数有关[10-11]。
本实验发现,与对照组相比,4%~8%酒精暴露组的RRDA随酒精浓度增加而逐渐减弱,TI缩短、RF下降、IA降低。提示孕期酒精暴露对RRDA具有浓度依赖性抑制作用。孕期酒精暴露抑制RRDA的机制可能与以下几方面相关:首先,孕期酒精暴露改变子代中枢神经系统神经元细胞膜受体表达,通过增加抑制性受体表达减少兴奋性受体来降低神经元兴奋性:胎儿在母体内接触酒精降低兴奋性受体NMDA-2B受体[12]和5-HT2A受体表达[13];孕期酒精暴露增加GABA受体活性并增加其表达[14-15]。以上各受体均参与延髓呼吸的发生和调节[16-18],孕期酒精暴露通过抑制呼吸兴奋性受体,激活抑制性受体,从而抑制延髓呼吸中枢功能,我们的实验结果也与上述文献一致。其次,酒精及其代谢产物还能通过离子通道来发挥作用,如降低Na+/K+-ATP酶[19]和Ca2+通道[20]的活性,而Na+内流和K+外流是动作电位形成的基础,钙离子是重要的生物信号传导分子,调控细胞的理化活动。再次,孕期酒精暴露可降低脑源性神经生长因子BDNF浓度,BDNF在神经元的存活和突触形成中起重要作用,其浓度的下降影响了神经元活性,降低神经网络功能[21-22]。另外,孕期酒精暴露使胎儿脑内线粒体发育迟缓,功能受损,ATP供给不足,从而影响了神经元活性及功能[23]。有研究表明,孕期酒精暴露能降低子代大鼠的每分钟通气量[4],而这极可能与孕期酒精暴露抑制新生大鼠延髓基本节律性呼放电有关。
综上所述,本研究结果表明,孕期酒精暴露抑制新生大鼠延髓脑片基本节律性呼吸放电活动,其机制可能是酒精通过诱导呼吸相关性受体、离子通道、神经生长因子及线粒体等发生改变,进而促使呼吸相关性神经元的活性下降和功能紊乱。孕期酒精暴露抑制新生大鼠延髓脑片基本节律性呼吸放电,这可能是孕期酒精暴露抑制子代整体水平呼吸功能的基础。
| [1] | Zhang FT, Wu ZH, Li YR. Effect of blocking medial area of nucleus retrofacialis on respiratory rhythm[J]. Respir Physiol,1991, 85 : 73-81. DOI: 10.1016/0034-5687(91)90007-6. |
| [2] | 吴中海, 张枫桐, 徐小元. 延髓面神经后核内侧区呼吸相关神经元的放电形式[J]. 生理学报,1997, 49 (4) : 389-94. |
| [3] | Astley SJ. The value of a FASD diagnosis[J]. Popul Ther Clin Pharmacol,2014, 21 (1) : e81-e105. |
| [4] | Dubois C, Houchi H, Naassila M, et al. Blunted response to low oxygen of rat respiratory network after perinatal ethanol exposure: involvement of inhibitory control[J]. Physiol,2008, 586 (5) : 1413-27. DOI: 10.1113/jphysiol.2007.147165. |
| [5] | Duncan JR, Randall LL, Belliveau RA, et al. The effect of maternal smoking and drinking during pregnancy upon (3) H-nicotine receptor brainstem binding in infants dying of the sudden infant death syndrome: initial observations in a high risk population[J]. Brain Pathol,2008, 18 (1) : 21-31. DOI: 10.1111/j.1750-3639.2007.00093.x. |
| [6] | Conradt E, Sheinkopf SJ, Lester BM, et al. Prenatal substance exposure: neurobiologic organization at 1 month[J]. J Pediatr,2013, 163 (4) : 989-94. DOI: 10.1016/j.jpeds.2013.04.033. |
| [7] | Suzue T. Respiratory rhythm generation in the in vitro brain-spinal cord preparation of the neonatal rat[J]. Physiol,1984, 354 : 173-83. DOI: 10.1113/jphysiol.1984.sp015370. |
| [8] | 齐莹, 千智斌, 吴中海. 组胺H1和H2受体在新生大鼠离体延髓脑片呼吸节律性放电调节中的作用[J]. 生理学报,2008, 60 (3) : 397-402. |
| [9] | Smith SM. Alcohol and cell death. In: Comprehensive Toxicology, McQueen CA, eds[M]. Oxford: Elesevier, 2010: 223-38. |
| [10] | Hutchinson D, Moore EA, Breen C, et al. Alcohol use in pregnancy: prevalence and predictors in the Longitudinal Study of Australian Children[J]. Drug Alcohol Rev,2013, 32 (5) : 475-82. |
| [11] | Stanfield KM, Wells JC, Fewtrell MS, et al. Differences in body composition between infants of South Asian and European ancestry: the London Mother and Baby Study[J]. Int J Epidemiol,2012, 41 (5) : 1409-18. DOI: 10.1093/ije/dys139. |
| [12] | Toso L, Poggi SH, Abebe D, et al. N-methyl-D-aspartate subunit expression during mouse development altered by in utero alcohol exposure[J]. Am J Obstet Gynecol,2005, 193 (4) : 1534-9. DOI: 10.1016/j.ajog.2005.02.105. |
| [13] | Kervern M, Dubois C, Naassila M, et al. Perinatal Alcohol Exposure in Rat Induces Long-Term Depression of Respiration after Episodic Hypoxia[J]. American Journal of Respiratory and Critical Care Medical,2009, 179 (7) : 608-14. DOI: 10.1164/rccm.200703-434OC. |
| [14] | Naseer MI, Lee HY, Ullah N, et al. siRNA-mediated GABA (B) receptor at early fetal rat brain upon acute and chronic ethanol exposure: down regulation of PKA and p-CREB expression[J]. Synapse,2011, 65 (2) : 109-18. DOI: 10.1002/syn.v65.2. |
| [15] | Werner DF, Kumar S, Criswell HE, et al. PKCγ is required for ethanol-induced increases in GABA (A) receptor α4 subunit expression in cultured cerebral cortical neurons[J]. Neurochem,2011, 116 (4) : 554-63. DOI: 10.1111/jnc.2011.116.issue-4. |
| [16] | Berger AJ. Development of synaptic transmission to respiratory motoneuron[J]. Respir Physiol Neurobiol,2011, 179 (1) : 34-42. DOI: 10.1016/j.resp.2011.03.002. |
| [17] | Niebert M, Vogelgesang S, Koch UR, et al. Expression and function of serotonin 2A and 2B receptors in the mammalian respiratory network[J]. PLoS One,2011, 6 (7) : e21395. DOI: 10.1371/journal.pone.0021395. |
| [18] | Janczewski WA, Tashima A, Hsu P, et al. Role of inhibition in respiratory pattern generation[J]. Neurosci,2013, 33 (13) : 5454-65. DOI: 10.1523/JNEUROSCI.1595-12.2013. |
| [19] | Reddy VD, Padmavathi P, Kavitha G, et al. Alcohol-induced oxidative/nitrosative stress alters brain mitochondrial membrane properties[J]. Mol Cell Biochem,2013, 375 (1-2) : 39-47. |
| [20] | Ma W, Pancrazio JJ, Andreadis JD, et al. Ethanol blocks cytosolic Ca2+ responses triggered by activation of GABA (A) receptor/Cl-channels in cultured proliferating rat neuroepithelial cells[J]. Neuroscience,2001, 104 (3) : 913-22. DOI: 10.1016/S0306-4522(01)00084-7. |
| [21] | Caldwell KK, Sheema S, Paz RD, et al. Fetal alcohol spectrum disorder-associated depression: evidence for reductions in the levels of brain-derived neurotrophic factor in a mouse model[J]. Pharmacol Biochem Behav,2008, 90 (4) : 614-24. DOI: 10.1016/j.pbb.2008.05.004. |
| [22] | Feng MJ, Yan SE, Yan QS. Effects of prenatal alcohol exposure on brain-derived neurotrophic factor and its receptor tyrosine kinase B in offspring[J]. Brain Res,2005, 1042 (2) : 125-32. DOI: 10.1016/j.brainres.2005.02.017. |
| [23] | Sari Y, Zhang M, Mechref Y. Differential expression of proteins in fetal brains of alcohol-treated prenatally C57BL/6 mice: a proteomic investigation[J]. Electrophoresis,2010, 31 (3) : 483-96. DOI: 10.1002/elps.v31:3. |
 2015, Vol. 35
2015, Vol. 35
