2. 南方医科大学第一临床医学院,广东 广州 510515 ;
3. 美国南加州大学洛杉矶儿童医院,洛杉矶 90027
2. First School of Clinical Medicine, Southern Medical University, Guangzhou 510515, China ;
3. Saban Research Institute, Children's Hospital Los Angeles, Los Angeles, CA 90027, USA
新生隐球菌B4500FO2、敲除CPS1基因的突变株TYCC645#32及回补株PCIP由本室保存,30 ℃培养在YPD肉汤中。收集对数期细胞,PBS洗2次,用血球计数板计数,调整菌液浓度108/ml重悬于含5% FBS的Hams-F12/M199培养基中备用。人脑微血管细胞(HBMEC)购于Sciencell公司,人白血病单核粒细胞(THP-1)购于上海中科院细胞库,37 ℃、5% CO2培养在含10% FBS的1640培养基中。
1.2 主要试剂和器材抗CD44单抗购自Santa Cruz公司;尿胰蛋白酶Bikunin购自北京鼎国昌盛生物技术有限责任公司;细胞膜荧光染料(PKH26 Red)购自Sigma公司;趋化小槽Millicell 12 μm购自Millipore公司;24、96孔板购自Corning公司;跨上皮细胞电阻测定仪(Millicell-Electrical Resistance System)为Millipore公司产品。
1.3 THP-1荧光染色室温下用无血清1640培养基清洗THP-1细胞1次,加入浓度为4×10-6 mol/ml PKH26染料中,放置2~5 min,轻摇混匀,保证充分混合,加入等量血清,终止染色反应,再放置1 min,离心去上清,1640培养基洗3次备用。
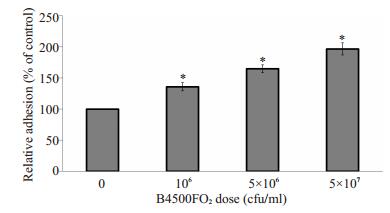
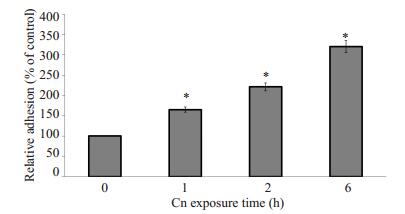
1.4 THP-1黏附分析将HBMEC调整浓度至5×105/ml,200 μl/孔铺于96孔板,37 ℃、5% CO2培养12 h。与不同菌株作用后,每孔加入PKH26染过色的浓度为2×106/ml的THP-1细胞200 μl,相同条件下孵育1 h后1640培养基清洗细胞3次,然后可在荧光显微镜下拍照计数,每个孔随机3个视野,实验重复3次,使用方差分析统计数据(ANOVA)。为观察不同剂量Cn B4500FO2刺激HBMEC对THP-1黏附率的影响,在加入THP-1之前,先分别加入200 μl以Hams-F12/M199培养基稀释的对数生长期、浓度为0、106、5 × 106和5 × 107 cfu/ml的B4500FO2株,37 ℃、5% CO2分别与HBMEC孵育,3 h后去除Cn,然后再添加THP-1孵育;为分析THP-1黏附HBMEC随Cn刺激时间变化的关系,同样方法,每孔加入5×106 cfu/ml浓度的Cn B4500FO2 200 μl,分别于1、2、6 h后去除Cn并冲洗细胞,再加入THP-1黏附HBMEC。
为观察Bikunin和anti-CD44单抗与HBMEC表达的CD44分子互作及B4500FO2刺激下的THP-1黏附率,以无血清1640培养基稀释Bikunin为0.1、1、5、20 nmol/L,并设PBS对照组,在Cn孵育HBMEC 3 h后冲洗培养板,Bikunin随THP-1一起加入96孔板孵育1 h;另一96孔板中各浓度组anti-CD44单抗加入量分别为每孔10、100、500、1000 ng。
分别使用浓度为5×106 cfu/ml的CPS1基因敲除株TYCC645#32、回补株CPIP和野生株B4500FO2刺激HBMEC,并设空白对照组,观察HA表达差异的不同菌株对THP-1迁移的作用。
1.5 构建体外血脑屏障模型12 μm膜孔径的趋化小槽Millicell对应24孔板使用,将培养5 d的HBMEC胰酶消化,调整浓度至2×105/ml,加400 μl至上槽中,再加600 μl含10% FBS的1640培养基至下槽中,37 ℃、5% CO2培养3~5 d,适时更换培养基。从第3天起用跨上皮细胞电阻测定仪测量跨膜电阻,当TEER值在200~300 Ω·cm2之间时可用来做趋化实验。
1.6 THP-1迁移分析与黏附实验一样,用不同的菌株孵育趋化小槽中的HBMEC 3 h后,轻柔冲洗小槽3次,在上槽内加入含2× 106个THP-1细胞的含10% FBS的1640培养基共400 μl,同时下槽液更换600 μl含10% FBS的1640培养基,让THP-1在小槽内做趋化运动后,在特定时间点取下槽液至血球计数板计数迁移至下槽液的THP-1细胞数,每孔重复3次,使用方差分析统计数据[20](ANOVA)。将实验后趋化小槽半透膜揭下,细胞刮板刮除上表面细胞,然后95%酒精固定过夜,0.1%结晶紫染色20 min,自来水漂洗2 min,显微镜拍摄整个半透膜[21]。
1.7 统计学处理所有实验至少重复3次,以均数±标准差表示数据,方差分析并以t检验或球对称检验来确定对照组和实验组之间差异。P < 0.05表明有统计学意义。
2 结果 2.1 Cn B4500FO2刺激HBMEC后对THP-1黏附迁移的影响THP-1的黏附与作用于HBMEC的Cn剂量呈正相关性,同时随Cn作用的时间延长而黏附率增加(图 1和图 2)。以PBS作为空白对照组孵育HBMEC 1 h时后的THP-1黏附率作为基数100%,Cn剂量在106 cfu/ml时黏附率相比是基数的136%,这时Cn与HBMEC细胞数量比约为2:1;当Cn剂量提高到5 × 107 cfu/ml时,THP-1黏附率就提高到基数197%,这时的Cn细胞数约是HBMEC的100倍。Cn作用于HBMEC的时间长短对THP-1黏附内皮细胞的影响更大,在1 h时只是基数的165%,而到6 h后,黏附率大幅提高到了320%。
|
图 1 Cn剂量对THP-1黏附HBMEC的影响 Figure 1 Dose-dependent induction of adhesion of THP-1 to HBMEC infected with different concentrations of Cn. Results are expressed as relative THP-1 adhesion compared with the PBS control (100%). Error bars indicate standard deviations. *P < 0.01 vs PBS control group. |
|
图 2 Cn刺激时间对THP-1黏附HBMEC的影响 Figure 2 Effect of Cn exposure time on adhesion THP-1 to HBMECs. Equivalent amount of wild strain B4500FO2 (5×106 cfu/ml) were incubated with HBMEC monolayer for 1, 2 and 6 h. Results are expressed as relative THP-1 adhesion compared with the PBS control (100%). *P < 0.01 vs PBS control group. |
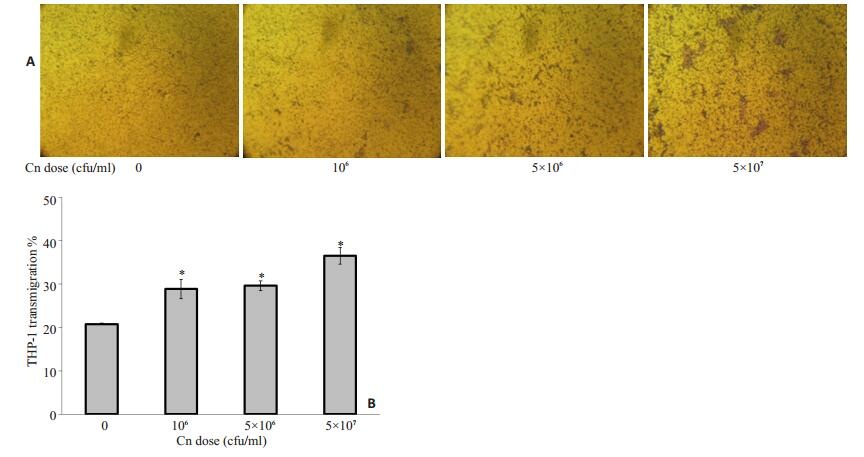
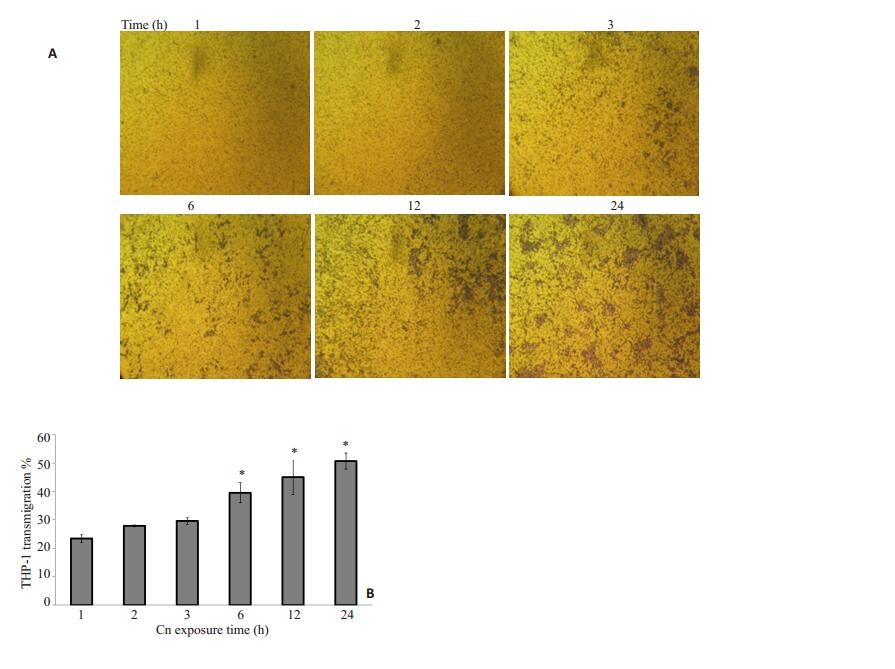
野生株Cn B4500FO2刺激HBMEC后明显增强了THP-1的趋化迁移(图 1~4)。实验表明,在Cn感染体外模型中的HBMEC时间(3 h)和THP-1加入小槽中迁移时间(3 h)都确定的情况下,Cn的剂量越大对THP-1迁移率的促进作用越强,相对于空白对照组20.8%的迁移率,106 cfu/ml时,迁移率28.875%;5×107 cfu/ml时,比没有Cn感染的情况下迁移率提高了75%还多。当Cn浓度为5 × 107 cfu/ml,与HBMEC作用时间为3 h,随THP-1在上槽液中时间延长,THP-1迁移率也相应提高。
|
图 3 Cn剂量对THP-1迁移率的影响 Figure 3 Effect of Cn dose on transmigration of THP-1 across HBMEC monolayer. A: HBMECs were transfected with scrambled or Cn at different doses followed by medium treatment for 3 h. The chemotactic activity was determined using a 24-well micro chemotaxis chamber. THP-1 cells were added to the upper chambers and allowed to migrate for 3 h through 12-μm porous membranes towards the lower chambers which contained the harvested conditioned media as indicated. Cells migrated to the lower chambers were fixed, stained, and photographed; B: Induction of THP-1 migration with different doses (0 to 5×107 cfu/ml) [corresponding to 0-100 multiplicity of infection (MOI)]of wild strain B4500FO2. *P < 0.01 vs control group (0 cfu/ml). |
|
图 4 Cn刺激时间对THP-1迁移率的影响 Figure 4 Effect of different Cnexposure time of HBMECs on transmigration of THP-1 cells. A: HBMECs were transfected with scrambled or Cn (5 × 106) followed by medium treatment for 1-24 h. THP-1 cells were added to the upper chambers and allowed to migrate for 3 h through 12-μm porous membranes towards the lower chambers containing the harvested conditioned media. B: Time-course study of Cn-induced THP-1 transmigration across HBMEC monolayer. THP-1 transmigration was triggered by 5 × 106 cfu/ml of wild strain B4500FO2. The values represent the mean percent transmigrating THP-1 of triplicate samples and are representative of one experiment from three independent experiments. *P < 0.01, **P < 0.05 vs control group. |
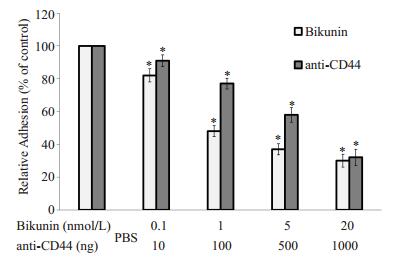
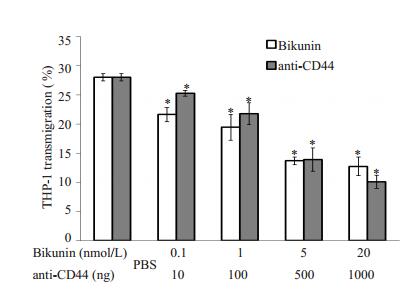
结果表明在5×107 cfu/ml B4500FO2感染HBMEC 3 h后,使用CD44抑制剂Bikunin或anti-CD44单克隆抗体孵育感染后的内皮细胞,都可以明显降低THP-1的黏附迁移作用(图 5、6),与只有Cn感染而不使用抗CD44药物的对照组相比,Bikunin和anti-CD44剂量越大,THP-1黏附率和迁移率就越低,使用抗CD44药物的最大剂量组的黏附率不及对照组的1/3,迁移率相比对照组降低了超过54%。
|
图 5 Bikunin和anti-CD44单克隆抗体剂量对THP-1黏附HBMEC的影响 Figure 5 THP-1 adhesion to HBMEC monolayers was blocked by Bikunin and anti-CD44 monoclonal antibody. Equivalentamount of wild strain B4500FO (2 5 × 107cfu/ml) were incubated with HBMEC monolayer for 3 h. HBMECs were then treated with different doses of bikunin and anti-CD44 monoclonal antibody, with the monolayers treated with Cn and PBS as the control. *P < 0.01 vs PBS control group. |
|
图 6 Bikunin和anti-CD44单克隆抗体剂量对THP-1迁移率的影响 Figure 6 Inhibition of THP-1 transmigration across HBMECs after preincubation with different doses of bikunin and anti-CD44 monoclonal antibody for 3 h. *P < 0.01 vs PBS control group. |
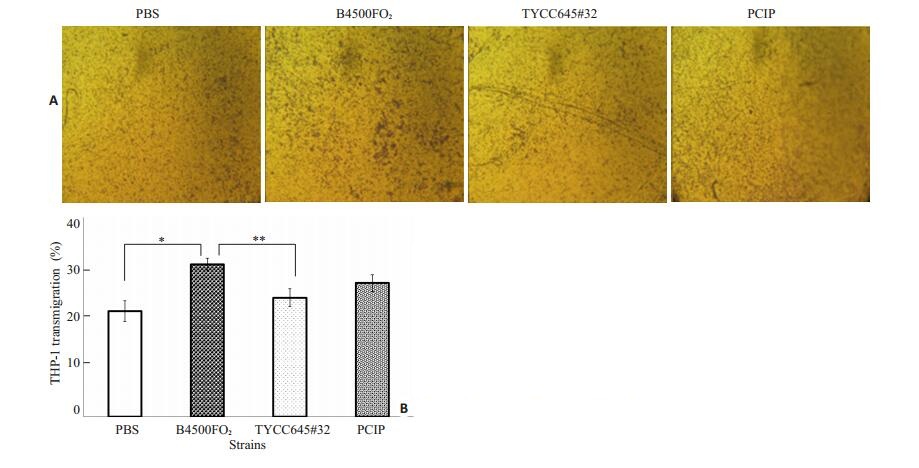
CPS1基因是Cn编码荚膜透明质酸HA合成酶的基因,敲除CPS1基因的TYCC645#32株,荚膜中不含HA,以Cn TYCC645#32(5×106 cfu/ml)和回补株PCIP(5×106 cfu/ml)分别感染HBMEC 3 h,野生株黏附率明显高于敲除株,而回补株相比敲除株黏附率有明显回升(图 7);迁移实验中,上槽液中加入400 μl浓度为5× 106/ml的THP-1,于3 h后计数下槽液中THP-1的迁移率,与PBS空白对照组比较THP-1的迁移率,CnB4500FO2(5×106 cfu/ml)组最高(图 8),与野生株组相比,敲除株TYCC645#32组则明显要低,但是回补株迁移率相比敲除株回升并不明显。

|
图 7 HA对THP-1黏附Cn感染的HBMEC的影响 Figure 7 Effect of HA on adhesion of THP-1 cells to HBMECs. Equivalent amount of wild strain B4500FO2, mutant TYCC645#32 and complemented strain CPIP were incubated with HBMEC monolayers for 3 h. Results are expressed as relative THP-1 adhesion compared with the PBS control (100%). Error bars indicate standard deviations.*P < 0.01 vs PBS control group. #P < 0.01. |
|
图 8 HA对THP-1穿越Cn感染的单层HBMEC的影响 Figure 8 Effect of HA on THP-1 transmigration across HBMEC after preincubation with different strains of Cn. A: HBMECs were transfected with scrambled or different Cn strains followed by medium treatment for 3 h; B: THP-1 transmigration rate. Equivalent amount of wild strain B4500FO2, mutant TYCC645#32 and complemented strain PCIP were incubated with HBMEC monolayers for 3 h. *P < 0.01, **P < 0.05. |
单核细胞迁移浸润是发生新生隐球菌脑膜炎时免疫系统抗抵抗感染的防御反应,Cn可以激活感染部位的内皮细胞产生趋化因子和促炎细胞因子[18],并引起脑微血管内皮细胞表面黏附分子表达改变,从而影响白细胞的招募和黏附迁移。为了明确Cn感染HBMEC后单核细胞通过血脑屏障的情况,我们通过黏附实验和迁移趋化实验,证明THP-1黏附率和迁移率随刺激HBMEC的Cn量的增加和刺激时间的延长而上升。HBMEC表达的PECAM-1(CD31)、E-Selectin和CD44等多种黏附分子参与其与白细胞的黏附作用,并且影响到白细胞通过血脑屏障的过程[19]。前期研究证实,Cn荚膜HA能够结合HBMEC表面的CD44,并引起宿主细胞的CD44在细胞膜上再聚集。本研究证实Cn感染微血管内皮细胞可以加强THP-1与HBMEC的黏附并提高THP-1通过单层HBMEC的迁移率。
CD44作为HA在HBMEC表面的主要受体,可被Bikunin和ant-CD44单克隆抗体抑制,实验结果显示,THP-1的黏附迁移作用随抑制剂或抗体浓度的增加而降低,可以证明CD44在THP-1黏附Cn刺激的HBMEC上起重要作用,并对白细胞迁移有影响。
CPS1基因敲除株TYCC645#32对比野生株B4500FO2,刺激HBMEC后对THP-1的黏附和迁移影响明显较低,而回补株PCIP则相对回升,表明Cn荚膜HA对THP-1黏附迁移也有影响,从而验证了作为HA受体的CD44在THP-1的黏附迁移中的重要作用。
综上所述,本研究首次证实新生隐球菌体外感染脑微血管内皮细胞可以诱导THP-1的黏附和迁移。前期研究中发现,Cn荚膜HA刺激HBMEC后,引起CD44表达的再分布,使单核细胞更容易通过HA结合,并激活引起细胞骨架和细胞形态的改变[7],从而使脑微血管内皮细胞之间的紧密连接发生改变,这两点可能是本研究中影响THP-1通过单层HBMEC的关键因素,说明HBMEC表达的CD44在单核细胞迁移通过血脑屏障中起重要作用。本研究表明新生隐球菌体外感染脑微血管内皮细胞诱导THP-1黏附和迁移的作用可能与CD44分子密切相关。CD44抗体和Bikunin,尤其是后者作为CD44的抑制剂可以明显减少单核细胞的迁移率,在减低炎症反应方面有一定应用前景。上述结果为新生隐球菌脑膜炎时Cn促进炎症发生发展的机制的相关研究提供启示,为寻找有效防治中枢神经系统机会感染的新途径提供理论基础。
| [1] | Yc C, Jong A, Huang S, et al. CPS1, a homolog of the Streptococcus pneumoniae type 3 polysaccharide synthase gene, is important for the pathobiology of Cryptococcus neoformans[J]. Infect Immun,2006, 74 (7) : 3930-8. DOI: 10.1128/IAI.00089-06. |
| [2] | Jong A, Wu CH, Chen HM, et al. Identification and characterization of CPS1 as a hyaluronic acid synthase contributing to the pathogenesis of Cryptococcus neoformans infection[J]. Eukaryot Cell,2007, 6 (8) : 1486-96. DOI: 10.1128/EC.00120-07. |
| [3] | Jong A, Wu CH, Shackleford GM, et al. Involvement of human CD44 during Cryptococcus neoformans infection of brain microvascular endothelial cells[J]. Cell Microbiol,2008, 10 (6) : 1313-26. DOI: 10.1111/j.1462-5822.2008.01128.x. |
| [4] | Jong A, Wu CH, Gonzales-Gomez IA, et al. Hyaluronic acid receptor CD44 deficiency is associated with decreased cryptococcus neoformans brain infection[J]. J Biol Chem,2012, 287 (19) : 15298-306. DOI: 10.1074/jbc.M112.353375. |
| [5] | Goodison S, Urquidi V, Tarin D. CD44 cell adhesion molecules[J]. Mol Pathol,1999, 52 (4) : 189-96. DOI: 10.1136/mp.52.4.189. |
| [6] | Neame SJ, Uff CR, Sheikh H, et al. CD44 exhibits a cell type dependent interaction with triton X-100 insoluble, lipid rich, plasma membrane domains[J]. J Cell Sci,1995, 108 (Pt 9) : 3127-35. |
| [7] | Long M, Huang SH, Wu CH, et al. Lipid raft/caveolae signaling is required for Cryptococcus neoformans invasion into human brain microvascular endothelial cells[J]. J Biomed Sci,2012, 19 (1) : 19. DOI: 10.1186/1423-0127-19-19. |
| [8] | Suzuki M, Kobayashi H, Fujie M, et al. Kunitz-type protease inhibitor bikunin disrupts phorbol ester-induced oligomerization of CD44 variant isoforms containing epitope v9 and subsequently suppresses expression of urokinase-type plasminogen activator in human chondrosarcoma cells[J]. J Biol Chem,2002, 277 (10) : 8022-32. DOI: 10.1074/jbc.M108545200. |
| [9] | Huang SH, Long M, Wu CH, et al. Invasion of cryptococcus neoformans into human brain microvascular endothelial cells is mediated through the lipid rafts-endocytic pathway via the dual specificity tyrosine phosphorylation-regulated kinase 3(DYRK3)[J]. J Biol Chem,2011, 286 (40) : 34761-9. DOI: 10.1074/jbc.M111.219378. |
| [10] | Charlier C, Nielsen K, Daou S, et al. Evidence of a role for monocytes in dissemination and brain invasion by cryptococcus neoformans[J]. Infect Immun,2009, 77 (1) : 120-7. DOI: 10.1128/IAI.01065-08. |
| [11] | Wakahara K, Kobayashi H, Yagyu T, et al. Bikunin down-regulates heterodimerization between CD44 and growth factor receptors and subsequently suppresses agonist -mediated signaling[J]. J Cell Biochem,2005, 94 (5) : 995-1009. DOI: 10.1002/(ISSN)1097-4644. |
| [12] | Hollenbaugh D, Mischel-Petty N, Edwards CP, et al. Expression of functional CD40 by vascular endothelial cells[J]. J Exp Med,1995, 182 (1) : 33-40. DOI: 10.1084/jem.182.1.33. |
| [13] | Turley EA, Noble PW, Bourguignon L. Signaling properties of hyaluronan receptors[J]. J Biol Chem,2002, 277 (7) : 4589-92. DOI: 10.1074/jbc.R100038200. |
| [14] | Humbert M, Ying S, Corrigan C, et al. Bronchial mucosal expression of the genes encoding chemokines RANTES and MCP-3 in symptomatic atopic andnonatopic asthmatics: Relationship to the eosinophil active cytokines interleukin (IL)-5, granulocyte macrophage-colony-stimulating factor, and IL-3[J]. Am J Respir Cell Mol Biol,1997, 16 : 1-8. DOI: 10.1165/ajrcmb.16.1.8998072. |
| [15] | Sabiiti W, May RC, Pursall ER. Experimental models ofcryptococcosis[J]. Int J Microbiol,2011, 2012 : 626745. |
| [16] | Steinmann U, Borkowski J, Wolburg H, et al. Transmigration of polymorphnuclear neutrophils and monocytes through the human blood-cerebrospinal fluid barrier after bacterial infection in vitro[J]. J Neuroinflam,2013, 10 (1) : 31. |
| [17] | Srikanta D, Santiago-Tirado F H, Doering T L. Cryptococcus neoformans: historical curiosity to modern pathogen[J]. Yeast,2014, 31 (2) : 47-60. DOI: 10.1002/yea.v31.2. |
| [18] | 张宇, 桑晨, 庄逢源. 内皮细胞相关黏附分子的研究进展[J]. 细胞与分子免疫学杂志,2009, 25 (1) : 89-91. |
| [19] | Razakandrainibe R, Combes V, Grau GE. Crossing the wall: The opening of endothelial cell junctions during infectious diseases[J]. Int J Biochem Cell Biol,2013, 45 (7) : 1165-73. DOI: 10.1016/j.biocel.2013.03.010. |
| [20] | Che X, Chi F, Wang L, et al. Involvement of IbeA in meningitic Escherichia coli K1-induced polymorphonuclear leukocyte transmigration across brain endothelial cells[J]. Brain Pathol,2011, 21 (4) : 389-404. DOI: 10.1111/bpa.2011.21.issue-4. |
| [21] | Lin CI, Chen CN, Lin PW, et al. Lysophosphatidic acid regulates inflammation-related genes in human endothelial cells through LPA1 and LPA3[J]. Biochem Biophys Res Commun,2007, 363 (4) : 1001-8. DOI: 10.1016/j.bbrc.2007.09.081. |
 2015, Vol. 35
2015, Vol. 35
