Journal of Southern Medical University ›› 2025, Vol. 45 ›› Issue (3): 603-613.doi: 10.12122/j.issn.1673-4254.2025.03.18
Yang LIU1,2( ), Yiqing JIA2, Chengcheng LI1,2, Handing MAO2, Shuyuan LIU2(
), Yiqing JIA2, Chengcheng LI1,2, Handing MAO2, Shuyuan LIU2( ), Yi SHAN1,2(
), Yi SHAN1,2( )
)
Received:2024-11-06
Online:2025-03-20
Published:2025-03-28
Contact:
Shuyuan LIU, Yi SHAN
E-mail:1316655020@qq.com;liusydoc@163.com;nghicu@163.com
Yang LIU, Yiqing JIA, Chengcheng LI, Handing MAO, Shuyuan LIU, Yi SHAN. Dexmedetomidine attenuates heat stress-induced oncosis in human skeletal muscle cells by activating the Nrf2/Ho-1 pathway[J]. Journal of Southern Medical University, 2025, 45(3): 603-613.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2025.03.18
| Target name | Primer sequences | |
|---|---|---|
| Actin | F | TCCTCCTGAGCGCAAGTACTCC |
| R | CATACTCCTGCTTGCTGATCCAC | |
| Porimin | F | CTCGGAACAATGGGACTCGG |
| R | TATGTTTGCAGATGCCGCC | |
| Caspase-3 | F | ATGACATCTCGGTCTGGTA |
| R | CTTTAGAAACATCACGCATC | |
| Nrf2 | F | TCAGCGACGGAAAGAGTATGA |
| R | CCACTGGTTTCTGACTGGATGT | |
| HO-1 | F | CCTTCCCCAACATTGCCAGT |
| R | CTTGGCCTCTTCTATCACCCTC | |
| NQO1 | F | GCTGGTTTGAGCGAGTGTTC |
| R | CTGCCTTCTTACTCCGGAAGG | |
Tab.1 The sequences of primers
| Target name | Primer sequences | |
|---|---|---|
| Actin | F | TCCTCCTGAGCGCAAGTACTCC |
| R | CATACTCCTGCTTGCTGATCCAC | |
| Porimin | F | CTCGGAACAATGGGACTCGG |
| R | TATGTTTGCAGATGCCGCC | |
| Caspase-3 | F | ATGACATCTCGGTCTGGTA |
| R | CTTTAGAAACATCACGCATC | |
| Nrf2 | F | TCAGCGACGGAAAGAGTATGA |
| R | CCACTGGTTTCTGACTGGATGT | |
| HO-1 | F | CCTTCCCCAACATTGCCAGT |
| R | CTTGGCCTCTTCTATCACCCTC | |
| NQO1 | F | GCTGGTTTGAGCGAGTGTTC |
| R | CTGCCTTCTTACTCCGGAAGG | |
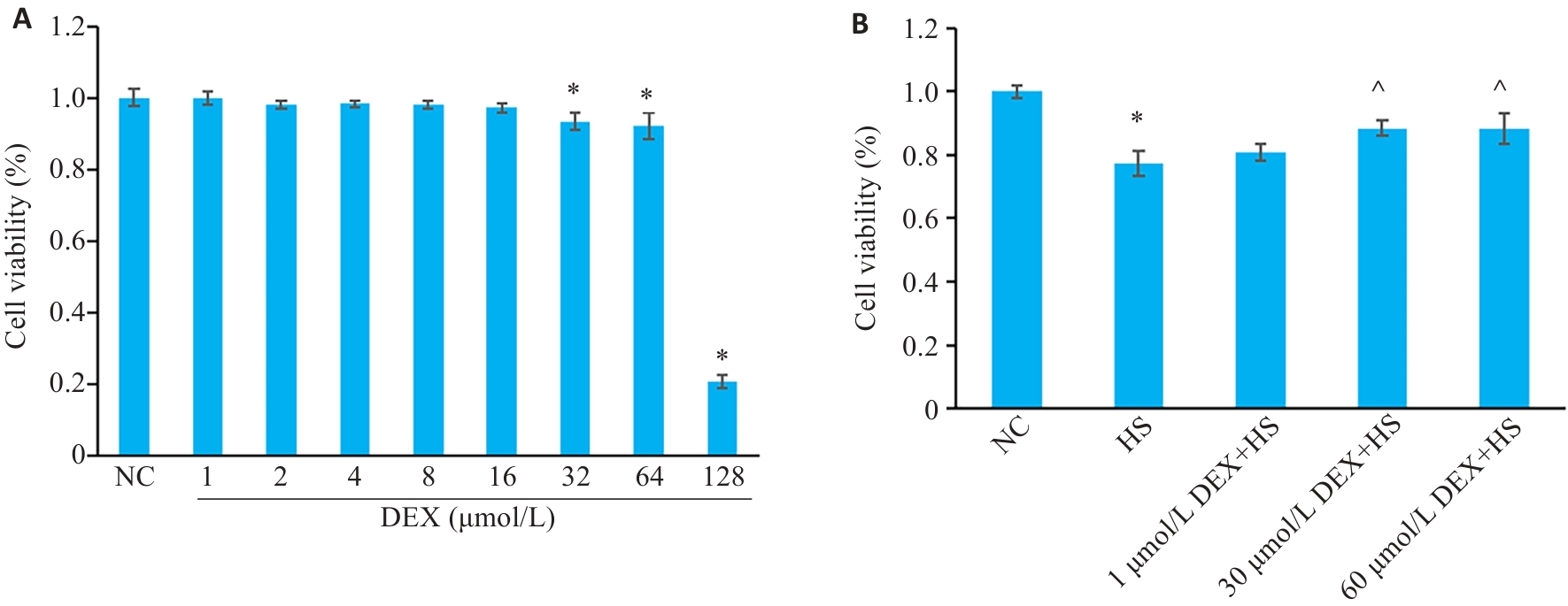
Fig.2 Screening of dexmedetomidine (DEX) dosing concentrations. A: Effects of different concentrations of DEX on HSKMC viability. *P<0.05 vs NC group (n=6). B: Effect of DEX (1, 30 and 60 μmol/L) on viability of HSKMC cells after heat stress. *P<0.05 vs NC group; ^P<0.05 vs HS group (n=6).
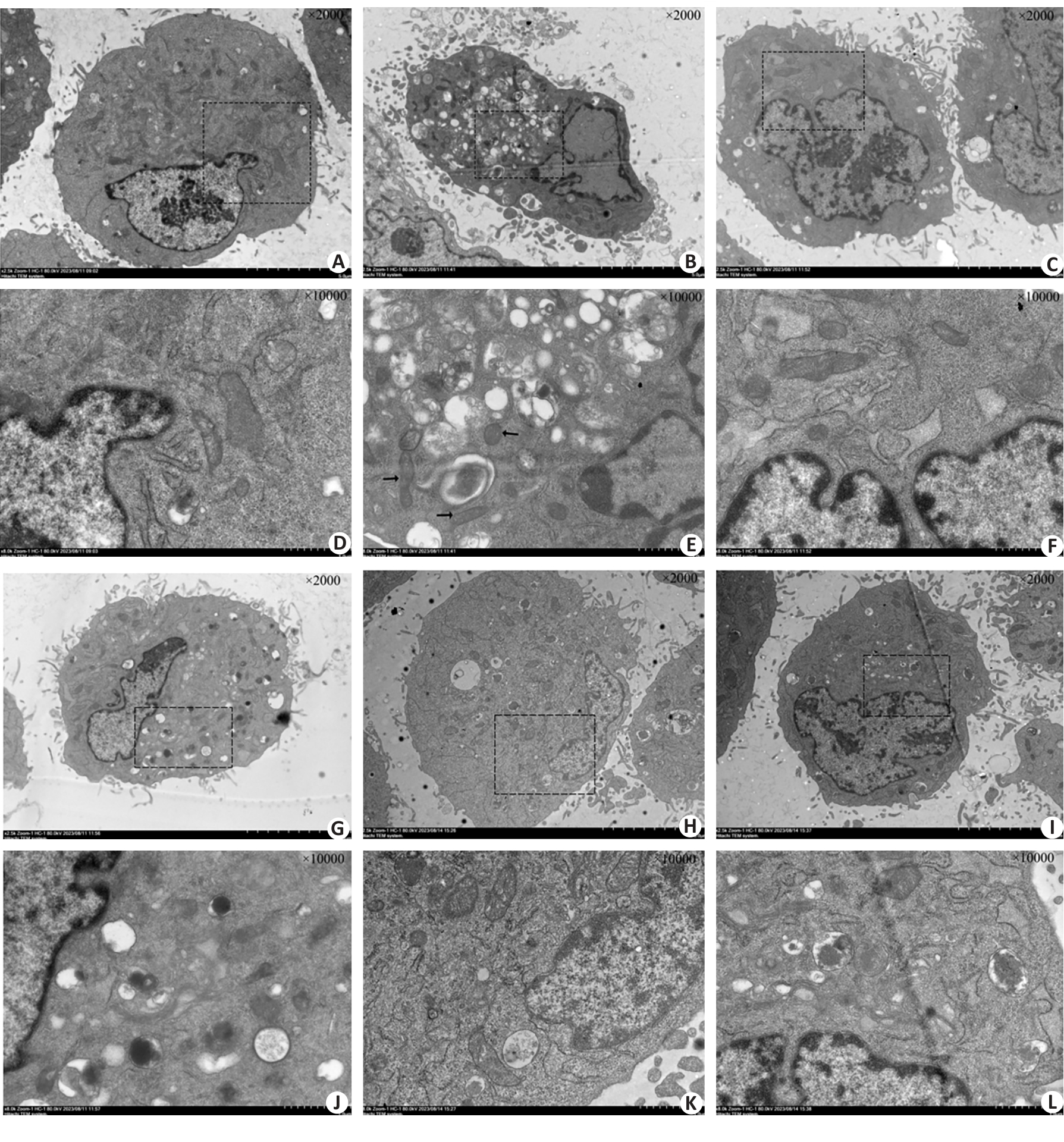
Fig.4 Observation of structural changes of HSKMCs under transmission electron microscope. Black arrows indicated mitochondrial swelling after heat stress. A: NC group (Original magnification: ×2000). B: HS group (×2000), C: 30 μmol/L DEX+HS group (×2000). D: NC group (×10 000). E: HS group (×10 000). F: 30 μmol/L DEX+HS group (×10 000). G: ML385+30 μmol/L DEX+HS group (×2000) . H: si-Nrf2+HS group (×2000). I: si-Nrf2+30 μmol/L DEX+HS group (×2000). J: ML385+30 μmol/L DEX+HS group (×10 000). K: si-Nrf2+HS group (×10 000) . L: si-Nrf2+30 μmol/L DEX+HS group (×10 000).
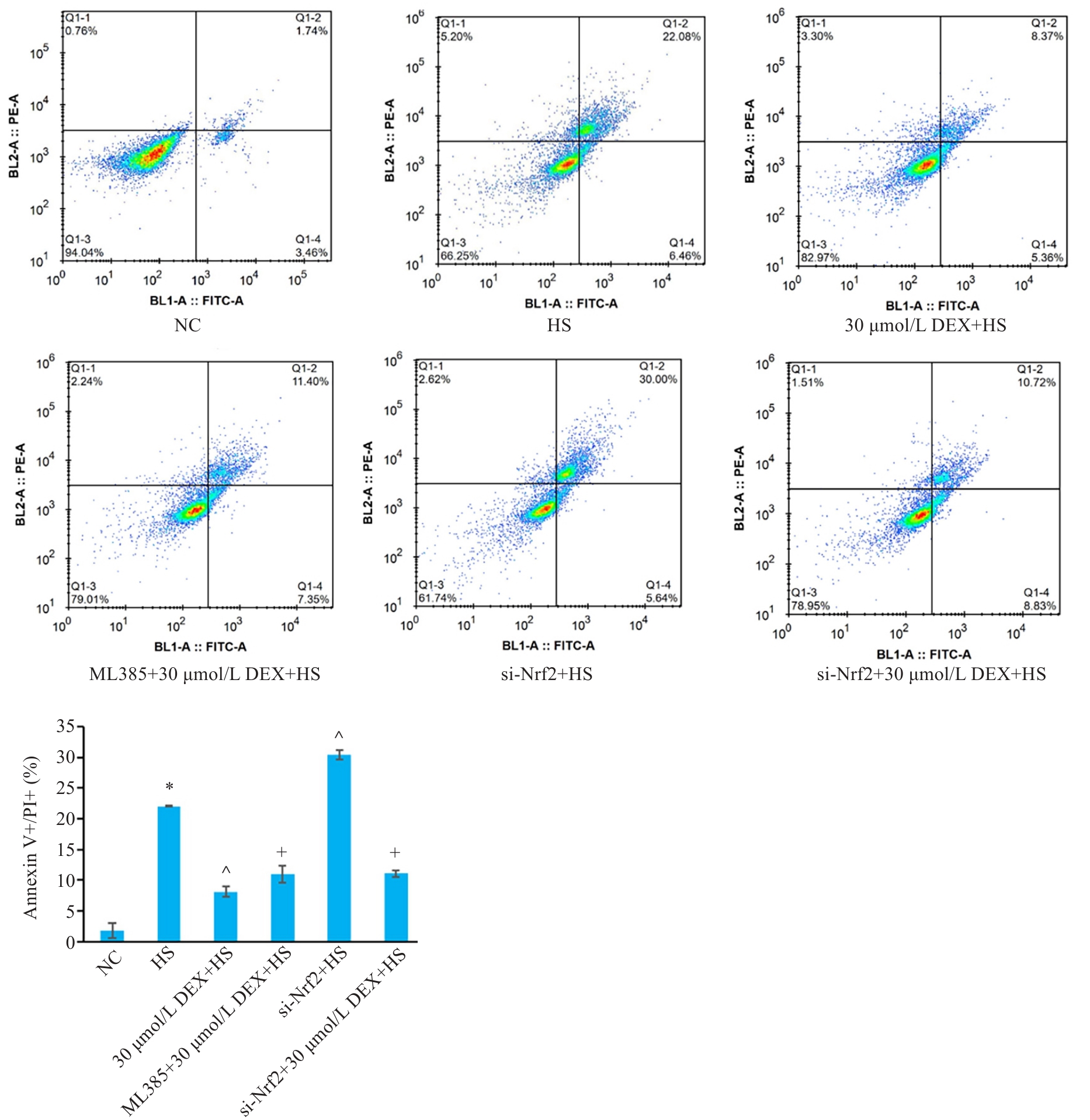
Fig.5 Comparison of Annexin-V+/PI+ double-positive cell rate in each group. *P<0.05 vs NC group; ^P<0.05 vs HS group; +P<0.05 vs 30 μmol/L DEX+HS group (n=3).
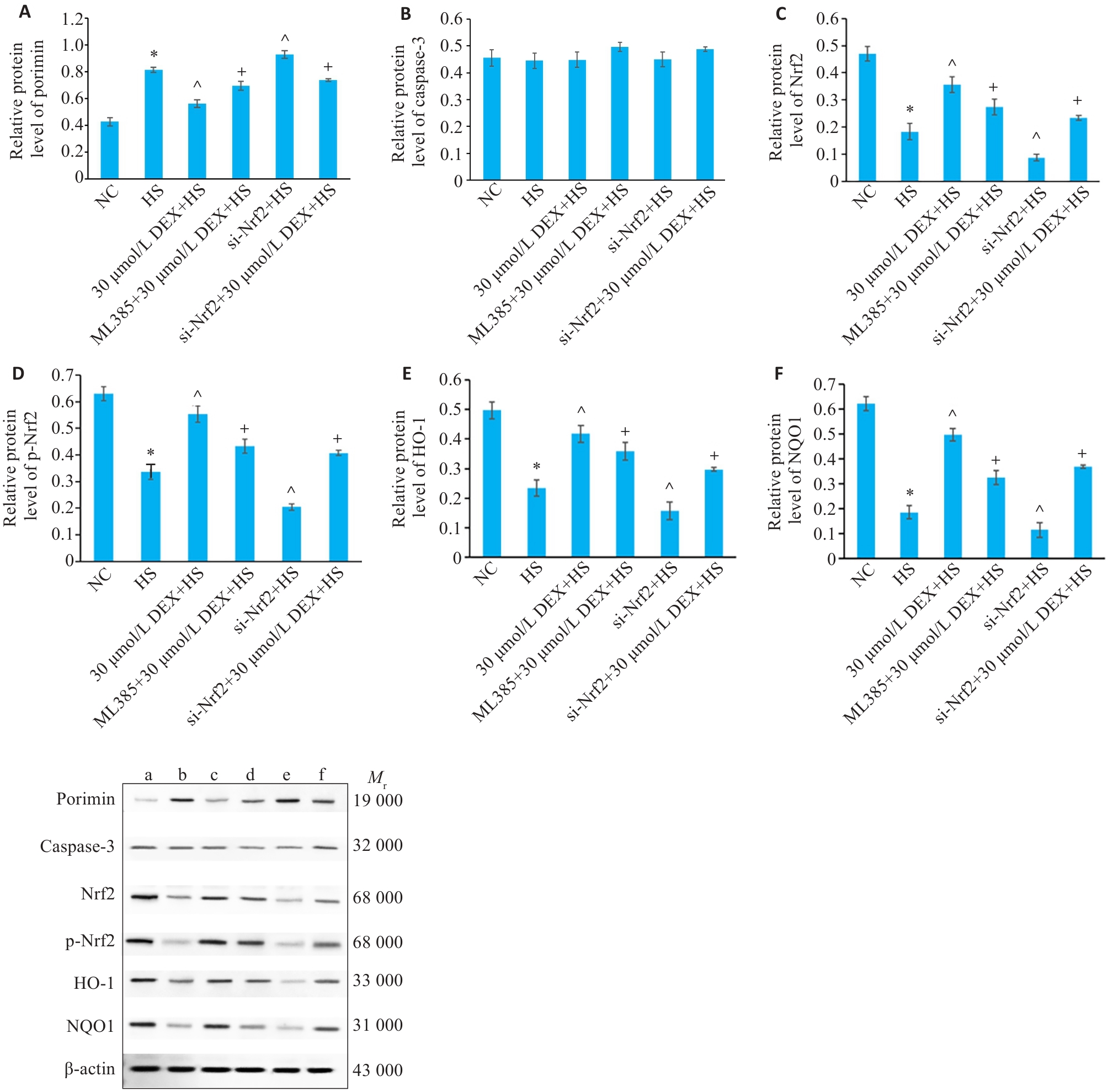
Fig.6 Protein expression levels of porimin (A), caspase-3 (B), Nrf2 (C), p-Nrf2 (D), HO-1 (E), and NQO1 (F) in HSKMCs in each group. *P<0.05 vs NC group; ^P<0.05 vs HS group; +P<0.05 vs 30 μmol/L DEX+HS group (Mean±SD, n=3). a: NC group; b: HS group; c: 30 μmol/L DEX+HS group; d: ML385+30 μmol/L DEX+HS group; e: si-Nrf2+HS group; f: si-Nrf2+DEX +HS group.
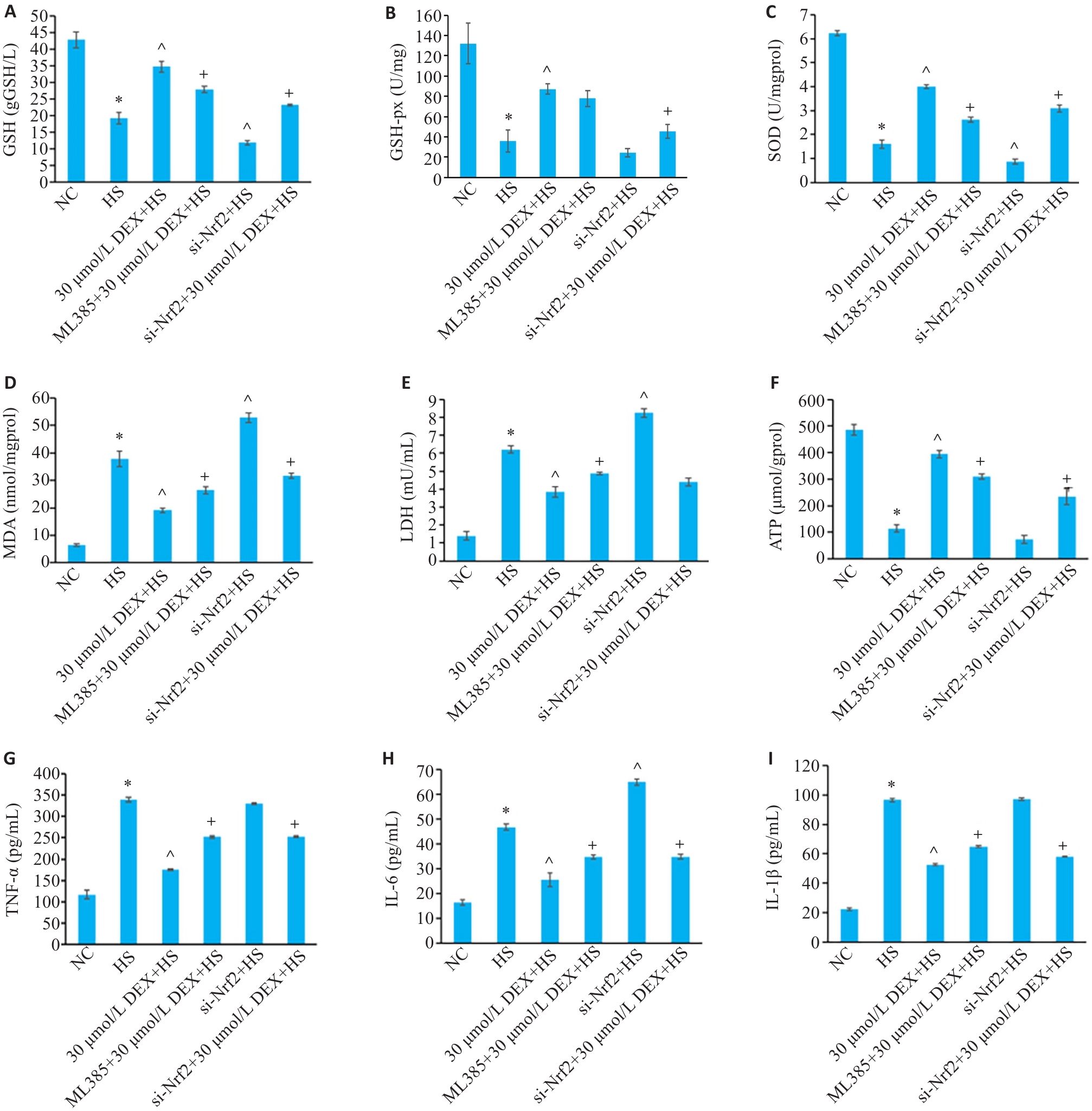
Fig.7 GSH (A), GSH-px (B), SOD (C), MDA (D), LDH (E), ATP (F), TNF-α (G), IL-6 (H), and IL-1β (I) levels in HSKMC in each group. *P<0.05 vs NC group; ^P<0.05 vs HS group; +P<0.05 vs 30 μmol/L DEX+HS group (Mean±SD, n=3).
| 1 | Torres PA, Helmstetter JA, Kaye AM, et al. Rhabdomyolysis: pathogenesis, diagnosis, and treatment[J]. Ochsner J, 2015, 15(1): 58-69. |
| 2 | Zutt R, van der Kooi AJ, Linthorst GE, et al. Rhabdomyolysis: review of the literature[J]. Neuromuscul Disord, 2014, 24(8): 651-9. |
| 3 | Cabral BMI, Edding SN, Portocarrero JP, et al. Rhabdomyolysis[J]. Dis Mon, 2020, 66(8): 101015. |
| 4 | Peters AA, Jamaludin SN, Yapa KS, et al. Oncosis and apoptosis induction by activation of an overexpressed ion channel in breast cancer cells[J]. Oncogene, 2017, 36(46): 6490-500. |
| 5 | Zheng JY, Tan HL, Matsudaira PT, et al. Excess reactive oxygen species production mediates monoclonal antibody-induced human embryonic stem cell death via oncosis[J]. Cell Death Differ, 2017, 24(3): 546-58. |
| 6 | Sun YX, Yu J, Liu XR, et al. Oncosis-like cell death is induced by berberine through ERK1/2-mediated impairment of mitochondrial aerobic respiration in gliomas[J]. Biomed Pharmacother, 2018, 102: 699-710. |
| 7 | Liu XM, Chen QH, Hu Q, et al. Dexmedetomidine protects intestinal ischemia-reperfusion injury via inhibiting p38 MAPK cascades[J]. Exp Mol Pathol, 2020, 115: 104444. |
| 8 | Weerink MAS, Struys MMRF, Hannivoort LN, et al. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine[J]. Clin Pharmacokinet, 2017, 56(8): 893-913. |
| 9 | Tian L, Liu Q, Wang X, et al. Fighting ferroptosis: Protective effects of dexmedetomidine on vital organ injuries[J]. Life Sci, 2024, 354: 122949. |
| 10 | Bao NR, Tang B. Organ-protective effects and the underlying mechanism of dexmedetomidine[J]. Mediators Inflamm, 2020, 2020: 6136105. |
| 11 | 李程程, 刘 洋, 毛汉丁, 等. 右美托咪定通过肾上腺素能α2受体调控NLRP3/IL-1β通路缓解劳力性热射病相关性横纹肌溶解[J/OL]. 解放军医学杂志, 2024: 1-14. |
| 12 | Qi YJ, Fu S, Pei DG, et al. Luteolin attenuated cisplatin-induced cardiac dysfunction and oxidative stress via modulation of Keap1/Nrf2 signaling pathway[J]. Free Radic Res, 2022, 56(2): 209-21. |
| 13 | Ulasov AV, Rosenkranz AA, Georgiev GP, et al. Nrf2/Keap1/ARE signaling: towards specific regulation[J]. Life Sci, 2022, 291: 120111. |
| 14 | D’Arcy MS. Cell death: a review of the major forms of apoptosis, necrosis and autophagy[J]. Cell Biol Int, 2019, 43(6): 582-92. |
| 15 | Lecoeur H, Prévost MC, Gougeon ML. Oncosis is associated with exposure of phosphatidylserine residues on the outside layer of the plasma membrane: a reconsideration of the specificity of the annexin V/propidium iodide assay[J]. Cytometry, 2001, 44(1): 65-72. |
| 16 | Zhou X, Sun WJ, Wang WM, et al. Artesunate inhibits the growth of gastric cancer cells through the mechanism of promoting oncosis both in vitro and in vivo [J]. Anticancer Drugs, 2013, 24(9): 920-7. |
| 17 | Duanghathaipornsuk S, Farrell EJ, Alba-Rubio AC, et al. Detection technologies for reactive oxygen species: fluorescence and electrochemical methods and their applications[J]. Biosensors, 2021, 11(2): 30. |
| 18 | 豆春丽. ROS介导线粒体功能障碍在热应激骨骼肌细胞胀亡中机制研究[D]. 广州: 广州中医药大学, 2020. |
| 19 | Peiris AN, Jaroudi S, Noor R. Heat stroke[J]. JAMA, 2017, 318(24): 2503. |
| 20 | Kodadek L, Carmichael Ii SP, Seshadri A, et al. Rhabdomyolysis: an American association for the surgery of trauma critical care committee clinical consensus document[J]. Trauma Surg Acute Care Open, 2022, 7(1): e000836. |
| 21 | Yue RC, Hu HX, Yiu KH, et al. Lycopene protects against hypoxia/reoxygenation-induced apoptosis by preventing mitochondrial dysfunction in primary neonatal mouse cardiomyocytes[J]. PLoS One, 2012, 7(11): e50778. |
| 22 | Chi XJ, Zhang R, Shen N, et al. Sulforaphane reduces apoptosis and oncosis along with protecting liver injury-induced ischemic reperfusion by activating the Nrf2/ARE pathway[J]. Hepatol Int, 2015, 9(2): 321-9. |
| 23 | Weerasinghe P, Maximilian Buja L. Oncosis: an important non-apoptotic mode of cell death[J]. Exp Mol Pathol, 2012, 93(3): 302-8. |
| 24 | Trump BF, Berezesky IK, Chang SH, et al. The pathways of cell death: oncosis, apoptosis, and necrosis[J]. Toxicol Pathol, 1997, 25(1): 82-8. |
| 25 | Wang YP, Gao WQ, Shi XY, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin[J]. Nature, 2017, 547(7661): 99-103. |
| 26 | Ge Y, Cai YM, Bonneau L, et al. Inhibition of cathepsin B by caspase-3 inhibitors blocks programmed cell death in Arabidopsis [J]. Cell Death Differ, 2016, 23(9): 1493-501. |
| 27 | 豆春丽, 袁芳芳, 刘 斌, 等. 热打击诱导骨骼肌细胞胀亡而非凋亡[J]. 解放军医学杂志, 2020, 45(10): 1047-51. |
| 28 | Wu C, Chen RL, Wang Y, et al. Acacetin alleviates myocardial ischaemia/reperfusion injury by inhibiting oxidative stress and apoptosis via the Nrf-2/HO-1 pathway[J]. Pharm Biol, 2022, 60(1): 553-61. |
| 29 | Park WH. Antiapoptotic effects of caspase inhibitors on H2O2-treated lung cancer cells concerning oxidative stress and GSH[J]. Mol Cell Biochem, 2018, 441(1/2): 125-34. |
| 30 | Zhang Q, Dong XW, Xia JY, et al. Obestatin plays beneficial role in cardiomyocyte injury induced by ischemia-reperfusion in vivo and in vitro [J]. Med Sci Monit, 2017, 23: 2127-36. |
| 31 | Yilmaz Y, Tumkaya L. Effects of hyperbaric oxygen and iloprost on intestinal ischemia-reperfusion induced acute lung injury[J]. Ann Surg Treat Res, 2019, 96(1): 34-40. |
| 32 | Yang L, Xing GL, Wang L, et al. Acute kidney injury in China: a cross-sectional survey[J]. Lancet, 2015, 386(10002): 1465-71. |
| 33 | Lankadeva YR, Ma S, Iguchi N, et al. Dexmedetomidine reduces norepinephrine requirements and preserves renal oxygenation and function in ovine septic acute kidney injury[J]. Kidney Int, 2019, 96(5): 1150-61. |
| 34 | Yao H, Chi XJ, Jin Y, et al. Dexmedetomidine inhibits TLR4/NF-κB activation and reduces acute kidney injury after orthotopic autologous liver transplantation in rats[J]. Sci Rep, 2015, 5: 16849. |
| 35 | Kopacz A, Kloska D, Forman HJ, et al. Beyond repression of Nrf2: an update on Keap1[J]. Free Radic Biol Med, 2020, 157: 63-74. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||