Journal of Southern Medical University ›› 2024, Vol. 44 ›› Issue (10): 1926-1936.doi: 10.12122/j.issn.1673-4254.2024.10.11
Previous Articles Next Articles
Zhifeng ZHOU1,2,3( ), Shuoyan LIU4, Jieyu LI1,2,3, Mingqiu CHEN5, Hui LIN4, Yujie CHEN4, Weijie CHEN4, Junpeng LIN4, Hang ZHOU4, Qinfeng ZHENG4(
), Shuoyan LIU4, Jieyu LI1,2,3, Mingqiu CHEN5, Hui LIN4, Yujie CHEN4, Weijie CHEN4, Junpeng LIN4, Hang ZHOU4, Qinfeng ZHENG4( )
)
Received:2024-01-11
Online:2024-10-20
Published:2024-10-31
Contact:
Qinfeng ZHENG
E-mail:zzf2004312 @fjzlhospital.com;zhqf@msn.com
Supported by:Zhifeng ZHOU, Shuoyan LIU, Jieyu LI, Mingqiu CHEN, Hui LIN, Yujie CHEN, Weijie CHEN, Junpeng LIN, Hang ZHOU, Qinfeng ZHENG. Modification with IL-21 and CCL19 enhances killing efficiency and tumor infiltration of NKP30 CAR-T cells in lung cancer[J]. Journal of Southern Medical University, 2024, 44(10): 1926-1936.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2024.10.11
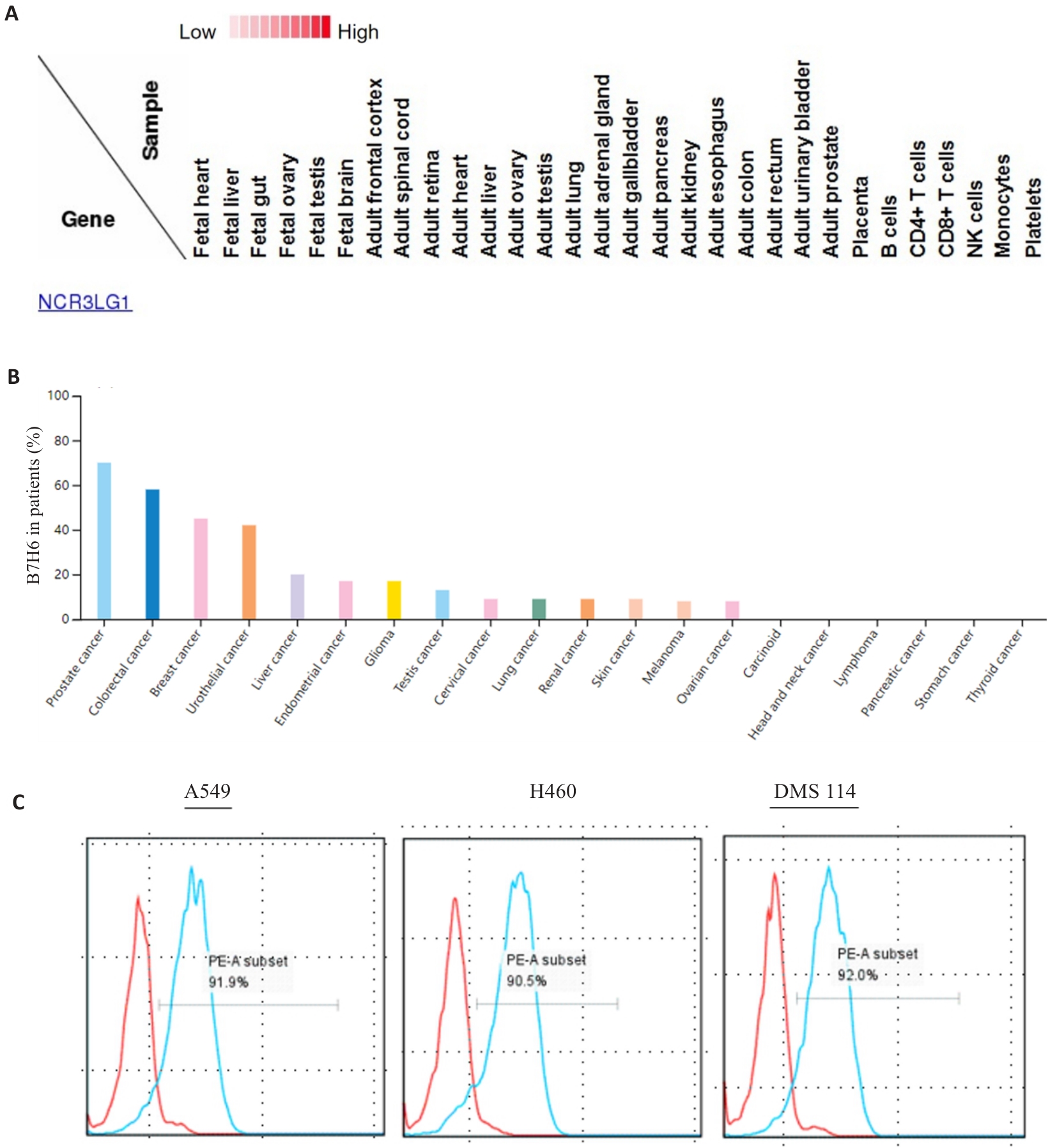
Fig.1 Expression of the NKP30 ligand B7H6 on the cells. A: Analysis of the Human Proteome Map database showing negative B7H6 expression in normal tissues. B: B7H6 is expressed in most of the tumor tissues. C: B7H6 is highly expressed in different lung cancer cell lines.
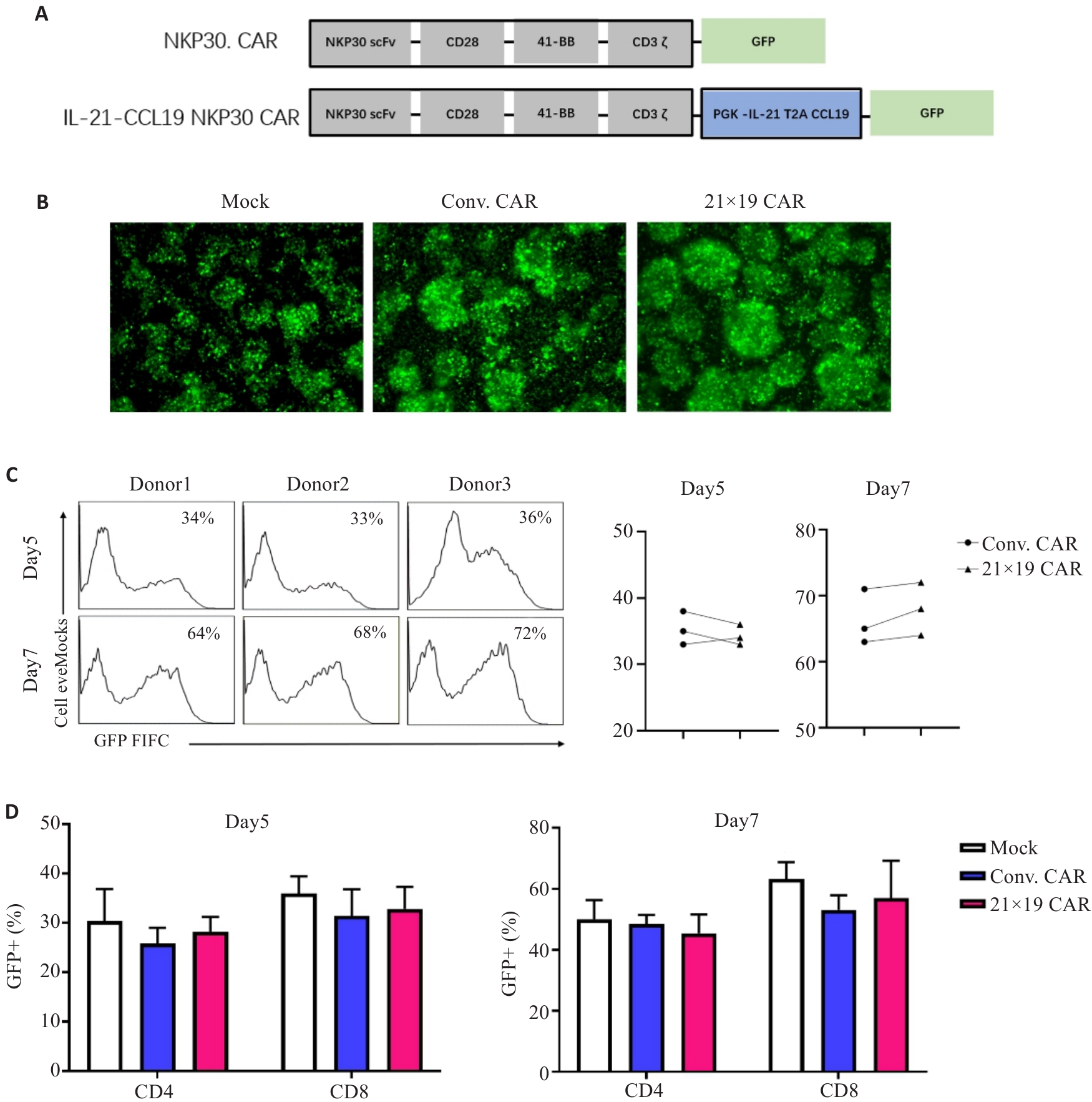
Fig.2 Construction of CAR and transfection of T cells. A: Schematic diagram of NKP30 CAR and IL-21-CCL19 NKP30 CAR. B: Fluorescence appeared in T cells 72 h after transfection (Original magnification: ×100). C: Results of CAR virus transfection in peripheral blood mononuclear cells from 3 donors, in which both Conv.CAR and 21×19 CAR achieved over 50% transfection after 7 days. D: Transfection efficiency of CAR-T cells on CD4+ and CD8+ T cells at different time points.
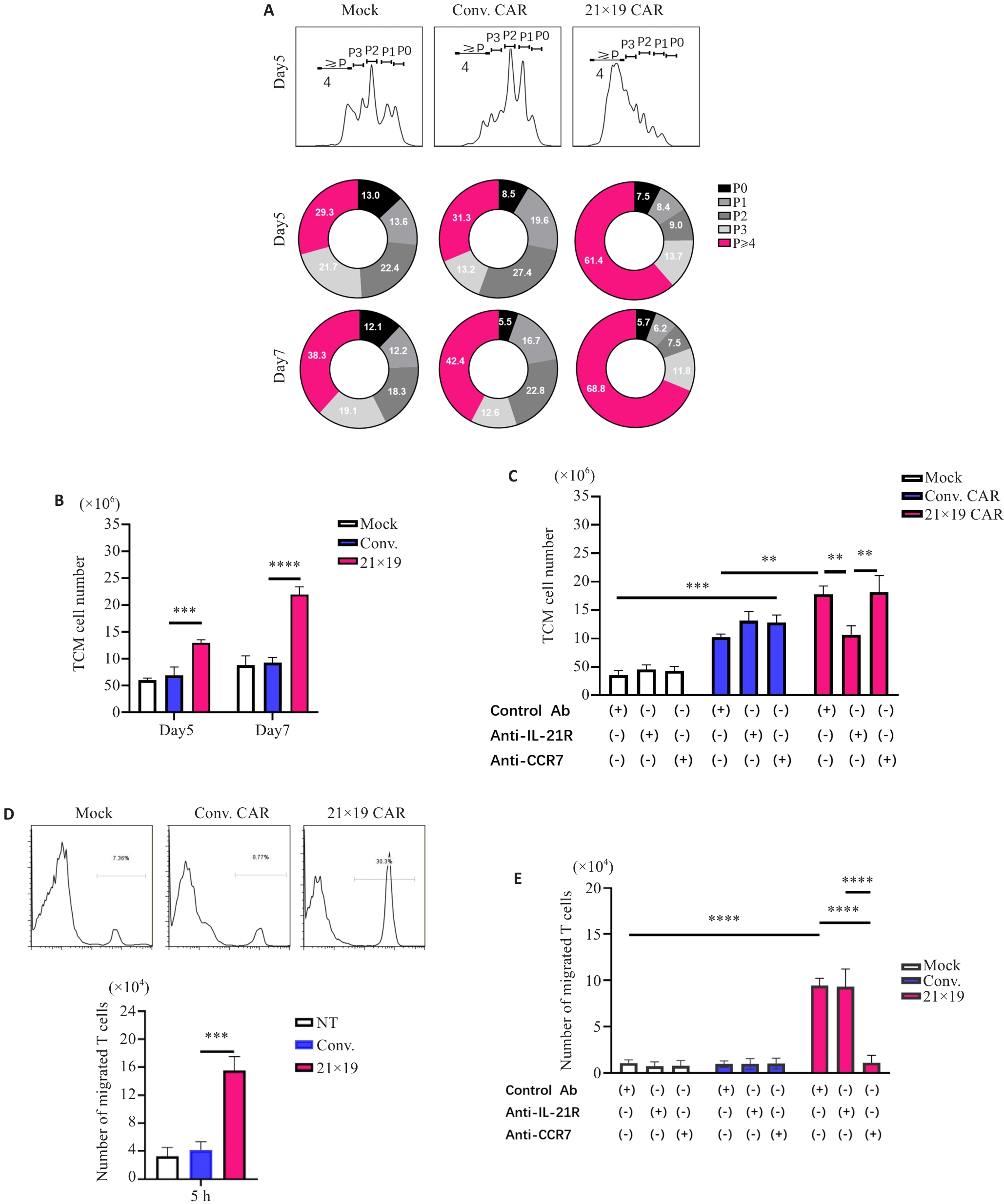
Fig.3 21×19 CAR-T promotes T cell proliferation and migration. A: Histograms of the numbers of cell divisions. The numbers in the donut charts represent percentages of each gated fraction in the cultured cells. B: Number of cells in each group on the 5th and 7th day. C: Anti-IL-21R monoclonal antibody down-regulates the formation of memory cells in 21×19 CAR-T group. D: Flow cytometry for analyzing chemotactic ability of the cells toward T cells in each group. E: Anti-CCR7 monoclonal antibody down-regulates the chemotactic ability of cells in 21×19 CAR-T group. **P<0.01, ***P<0.001, ****P<0.0001 by one-way ANOVA with multiple comparisons.
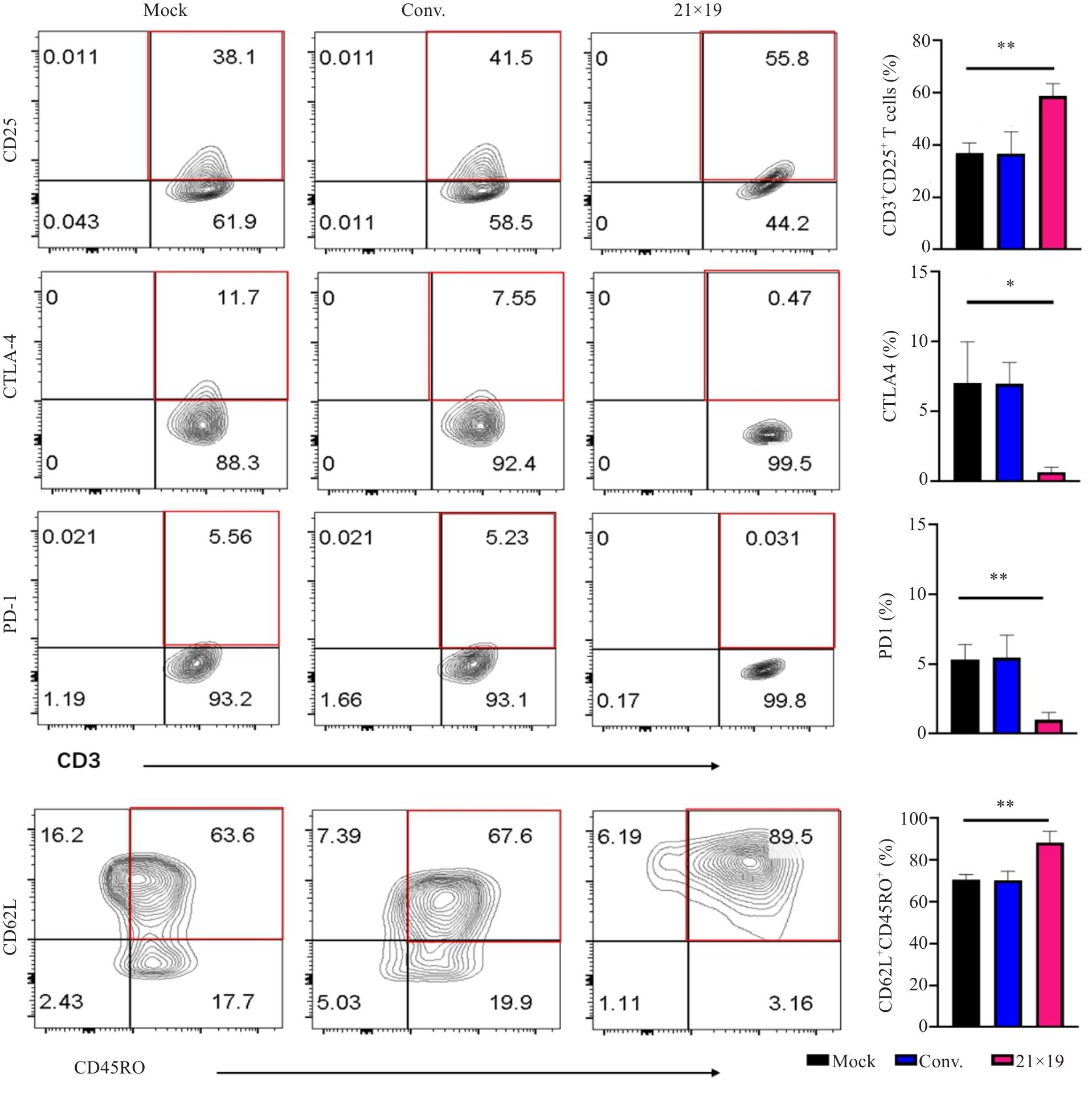
Fig.4 Phenotype of cultured CAR-T cells. 21×19 CAR-T cells expressed significantly higher levels of activation markers CD3/CD25 and central memory markers CD62L/CD45RO with lower CTLA4 and PD1 expressions than other cells. The red box indicates the positive region. *P<0.05, **P<0.01.
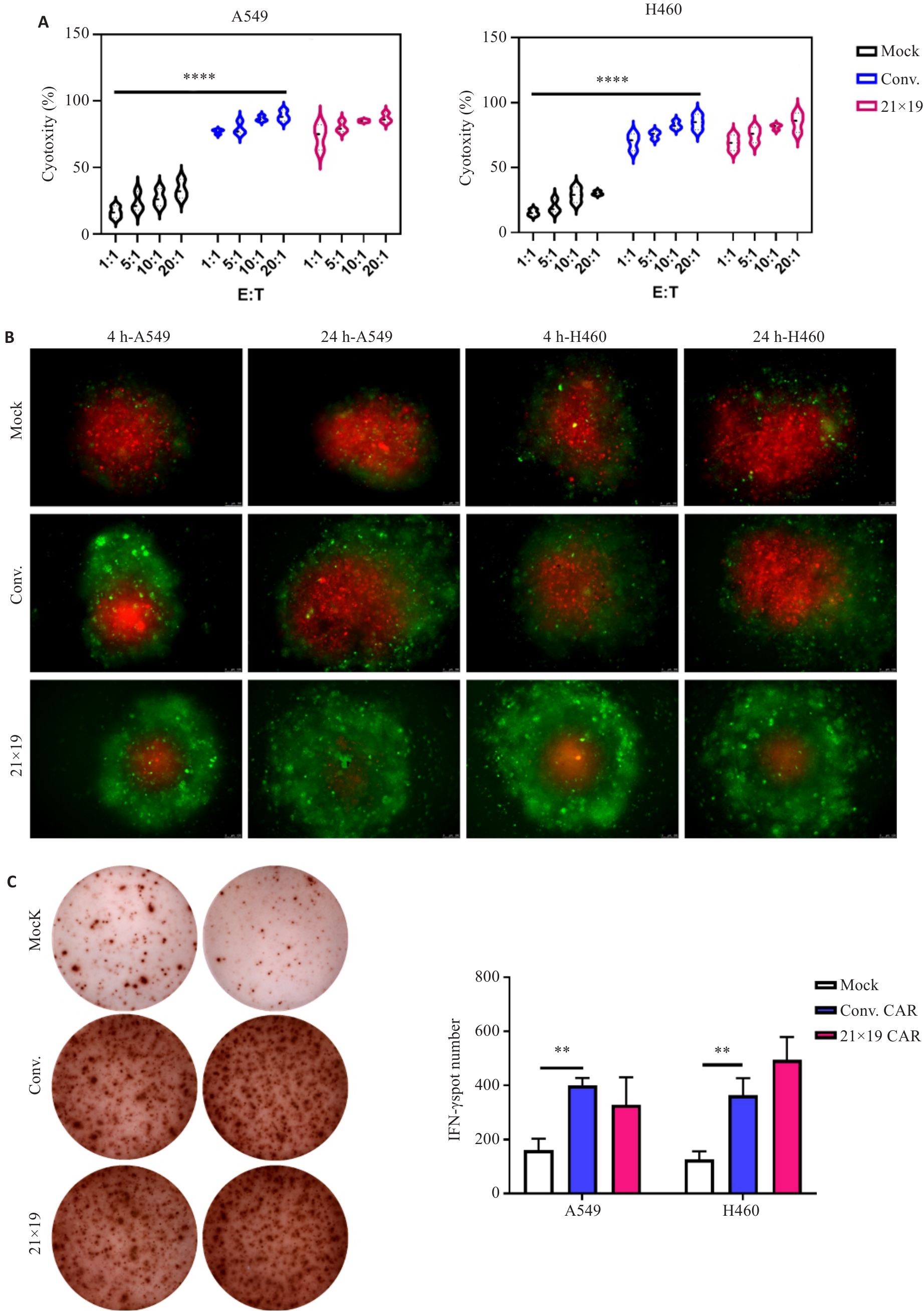
Fig.5 21×19 CAR show enhanced cytotoxic activity in vitro. A: LDH assay for assessing cytotoxic activity of immune cells from each group co-cultured with lung cancer cells at effector-to-target (E:T) ratios of 1:1, 5:1, 10:1, and 20:1. B: Immune cells from each group co-cultured with the target tumor cell spheroids at different time points (E:T=1:1). Green fluorescence represents the effector cells, and red fluorescence represent the tumor cells (×100). C: ELISPOT for measuring the amount of IFN-γ produced by the effector cells in response to lung cancer cells. **P<0.01, ****P<0.0001.
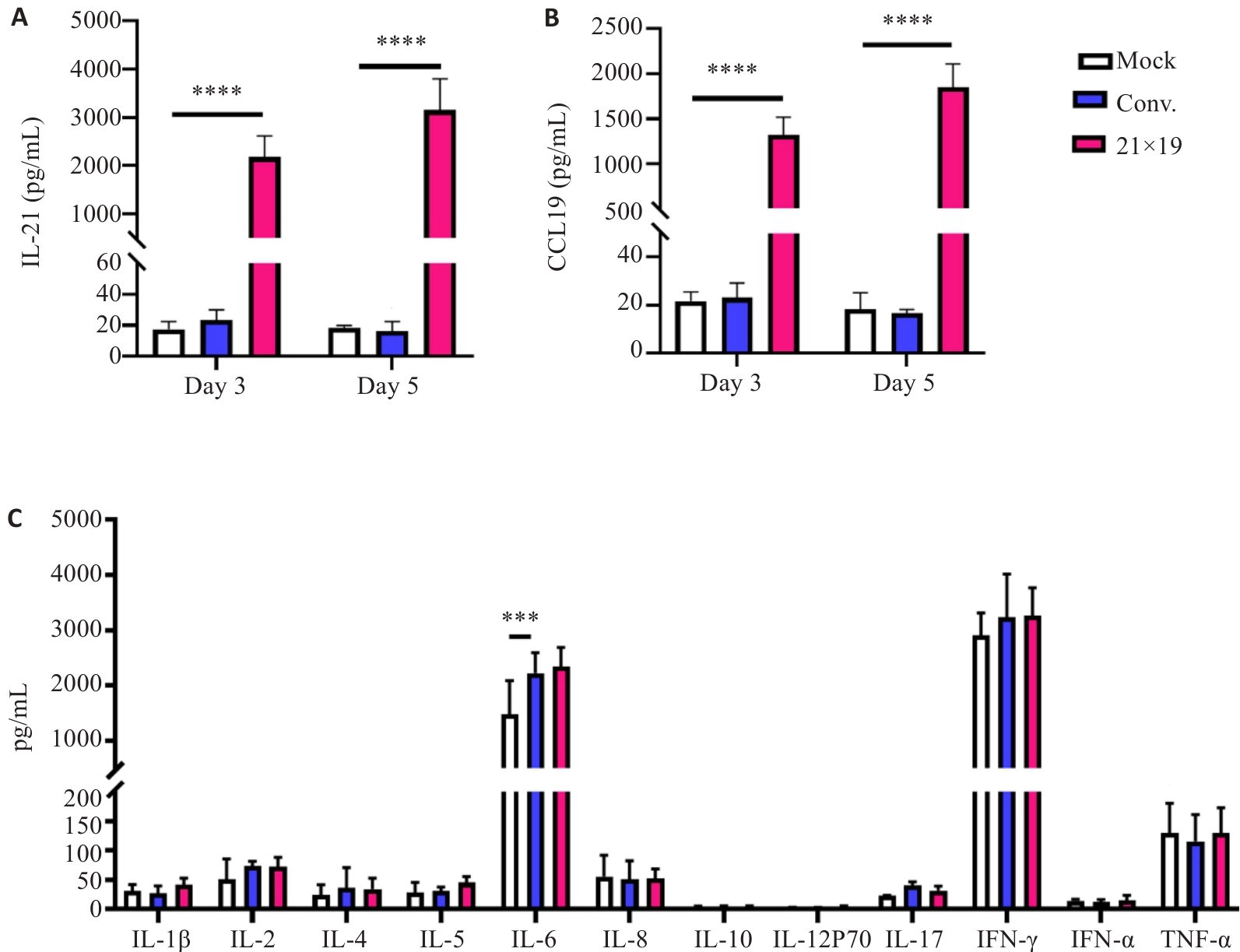
Fig.6 21×19 CAR-T cells can effectively secrete IL-21 and CCL19 cytokines. A, B: Compared with Mock cells and conventional CAR-T cells, 21×19 CAR-T cells secreted a large amount of IL-21 (A) and CCL-19 (B). C: Both CAR-T and 21×19 CAR-T cells produce higher IL-6 level than cells in the Mock group, there is no significant difference among the 3 groups. ***P<0.001, ****P<0.0001.
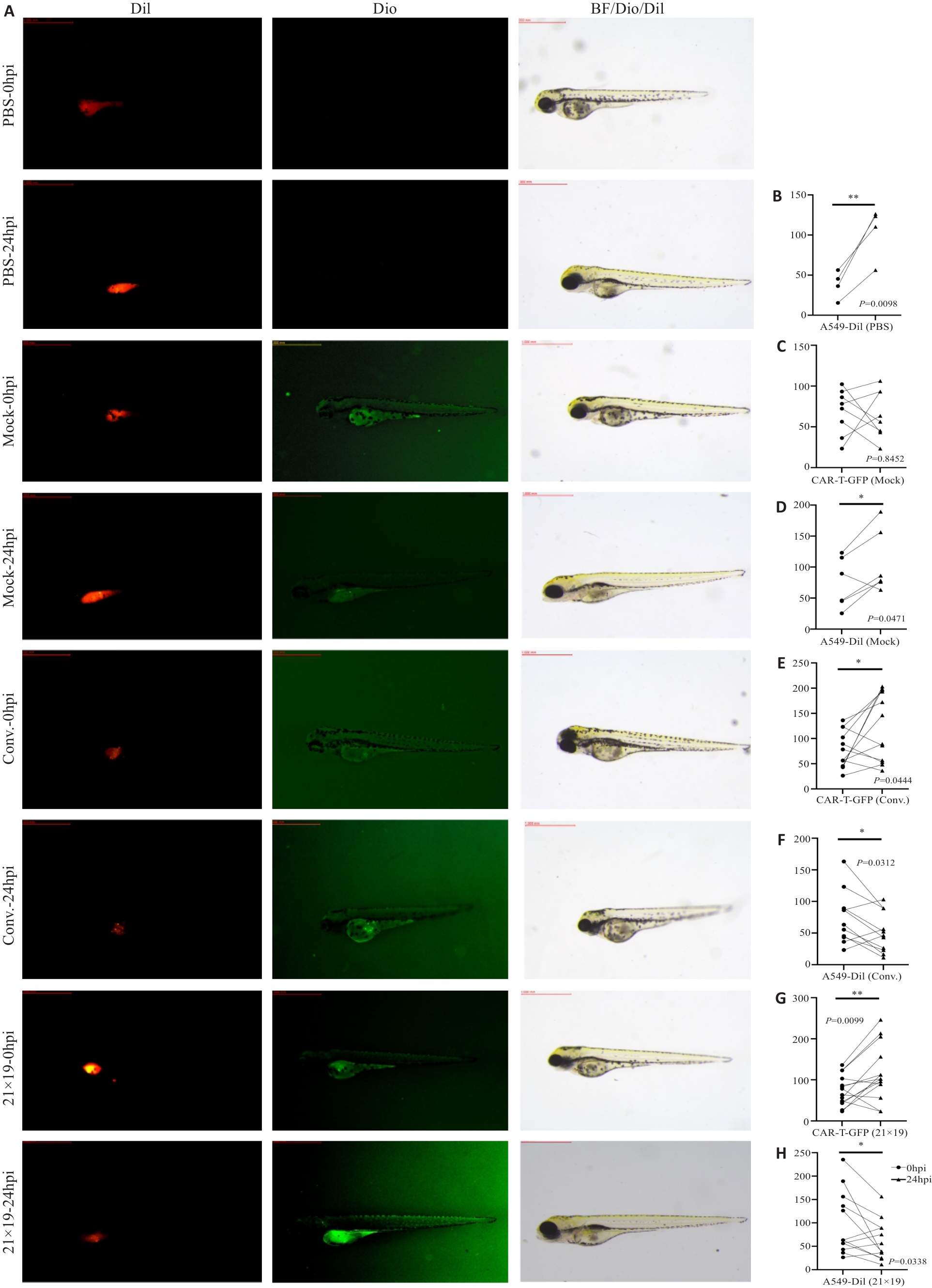
Fig. 7 CAR-T cells eliminate lung cancer cells in zebrafish. A: Fluorescence microscopy of the tumor-bearing zebrafish at 0 and 24 hpi time points (×30). The tumor cells A549 were stained with red fluorescent Dil, and the effector cells carry green fluorescent protein (GFP). B: Tumor volume increases in the zebrafish models 24 h after PBS injection. C, D: No significant area expansion of T cells and increased tumor volume in the Mock group after 24 h. E, F: In the conv. group, the area of CAR-T cells shows partial expansion with reduced tumor volume after 24 hours. G: In the 21×19 CAR-T group, the area of CAR-T cells significantly expands and the tumor volume is significantly reduced after 24 h. *P<0.05, **P<0.01.
| 1 | Liu YJ, He YY. A narrative review of chimeric antigen receptor-T (CAR-T) cell therapy for lung cancer[J]. Ann Transl Med, 2021, 9(9): 808. |
| 2 | Schubert ML, Schmitt M, Wang L, et al. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy[J]. Ann Oncol, 2021, 32(1): 34-48. |
| 3 | Cochrane RW, Fiorentino A, Allen E, et al. How to test human CAR T cells in solid tumors, the next frontier of CAR T cell therapy[M]// Cancer Immunotherapy. New York: Humana, 2024: 243-265. |
| 4 | Gutierrez-Silerio GY, Bueno-Topete MR, Vega-Magaña AN, et al. Non-fitness status of peripheral NK cells defined by decreased NKp30 and perforin, and increased soluble B7H6, in cervical cancer patients[J]. Immunology, 2023, 168(3): 538-53. |
| 5 | Mohammadi A, Najafi S, Amini M, et al. The potential of B7-H6 as a therapeutic target in cancer immunotherapy[J]. Life Sci, 2022, 304: 120709. |
| 6 | SHAEivary, Kheder RK, Najmaldin SK, et al. Implications of IL-21 in solid tumor therapy[J]. Med Oncol, 2023, 40(7): 191. |
| 7 | Ma MC, Xie YY, Liu JH, et al. Biological effects of IL-21 on immune cells and its potential for cancer treatment[J]. Int Immunopharmacol, 2024, 126: 111154. |
| 8 | Xu DH, Liu X, Ke SY, et al. CCL19/CCR7 drives regulatory T cell migration and indicates poor prognosis in gastric cancer[J]. BMC Cancer, 2023, 23(1): 464. |
| 9 | Gacerez AT, Hua CK, Ackerman ME, et al. Chimeric antigen receptors with human scFvs preferentially induce T cell anti-tumor activity against tumors with high B7H6 expression[J]. Cancer Immunol Immunother, 2018, 67(5): 749-59. |
| 10 | Silvestre RN, Eitler J, de Azevedo JTC, et al. Engineering NK-CAR.19 cells with the IL-15/IL-15Rα complex improved proliferation and anti-tumor effect in vivo [J]. Front Immunol, 2023, 14: 1226518. |
| 11 | Liu ZH, Zhou JY, Yang XZ, et al. Safety and antitumor activity of GD2-Specific 4SCAR-T cells in patients with glioblastoma[J]. Mol Cancer, 2023, 22(1): 3. |
| 12 | Morello A, Sadelain M, Adusumilli PS. Mesothelin-targeted CARs: driving T cells to solid tumors[J]. Cancer Discov, 2016, 6(2): 133-46. |
| 13 | Feng KC, Guo YL, Dai HR, et al. Chimeric antigen receptor-modified T cells for the immunotherapy of patients with EGFR-expressing advanced relapsed/refractory non-small cell lung cancer[J]. Sci China Life Sci, 2016, 59(5): 468-79. |
| 14 | Zeltsman M, Dozier J, McGee E, et al. CAR T-cell therapy for lung cancer and malignant pleural mesothelioma[J]. Transl Res, 2017, 187: 1-10. |
| 15 | Zeng R, Spolski R, Casas E, et al. The molecular basis of IL-21-mediated proliferation[J]. Blood, 2007, 109(10): 4135-42. |
| 16 | Ngai H, Tian GW, Courtney AN, et al. IL-21 selectively protects CD62L+ NKT cells and enhances their effector functions for adoptive immunotherapy[J]. J Immunol, 2018, 201(7): 2141-53. |
| 17 | Ptáčková P, Musil J, Štach M, et al. A new approach to CAR T-cell gene engineering and cultivation using piggyBac transposon in the presence of IL-4, IL-7 and IL-21[J]. Cytotherapy, 2018, 20(4): 507-20. |
| 18 | Singh H, Figliola MJ, Dawson MJ, et al. Reprogramming CD19-specific T cells with IL-21 signaling can improve adoptive immunotherapy of B-lineage malignancies[J]. Cancer Res, 2011, 71(10): 3516-27. |
| 19 | Pang NZ, Shi JX, Qin L, et al. IL-7 and CCL19-secreting CAR-T cell therapy for tumors with positive glypican-3 or mesothelin[J]. J Hematol Oncol, 2021, 14: 118. |
| 20 | Adachi K, Kano Y, Nagai T, et al. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor[J]. Nat Biotechnol, 2018, 36(4): 346-51. |
| 21 | Yao YX, Wang L, Wang X. Modeling of solid-tumor microenvironment in zebrafish (Danio rerio) larvae[M]// Tumor Microenvironment. Cham: Springer, 2020: 413-428. |
| 22 | Reinhardt F, Coen L, Rivandi M, et al. DanioCTC: analysis of circulating tumor cells from metastatic breast cancer patients in zebrafish xenografts[J]. Cancers, 2023, 15(22): 5411. |
| 23 | 殷 果, 李 荣, 刘岳飞, 等. Notch信号通路抑制剂DAPT改善酒精诱导的斑马鱼神经元分化障碍[J]. 南方医科大学学报, 2023, 43(6): 889-99. DOI: 10.12122/j.issn.1673-4254.2023.06.03 |
| 24 | Li Y, Liu WH, Xu J, et al. Chlorahololide D, a lindenane-type sesquiterpenoid dimer from Chloranthus holostegius suppressing breast cancer progression[J]. Molecules, 2023, 28(20): 7070. |
| 25 | Murali Shankar N, Ortiz-Montero P, Kurzyukova A, et al. Preclinical assessment of CAR-NK cell-mediated killing efficacy and pharmacokinetics in a rapid zebrafish xenograft model of metastatic breast cancer[J]. Front Immunol, 2023, 14: 1254821. |
| 26 | Barati M, Chahardehi AM, Hosseini Y. Finding integrative medication for neuroblastoma and glioblastoma through zebrafish as A model of organism[J]. Curr Top Med Chem, 2023, 23(30): 2807-20. |
| 27 | Pascoal S, Salzer B, Scheuringer E, et al. A preclinical embryonic zebrafish xenograft model to investigate CAR T cells in vivo [J]. Cancers, 2020, 12(3): 567. |
| 28 | Rejeski K, Subklewe M, Locke FL. Recognizing, defining, and managing CAR-T hematologic toxicities[J]. Hematology Am Soc Hematol Educ Program, 2023, 2023(1): 198-208. |
| 29 | Li XL, Gong NQ, Tian FL, et al. Suppression of cytokine release syndrome during CAR-T-cell therapy via a subcutaneously injected interleukin-6-adsorbing hydrogel[J]. Nat Biomed Eng, 2023, 7(9): 1129-41. |
| 30 | Stock S, Klüver AK, Fertig L, et al. Mechanisms and strategies for safe chimeric antigen receptor T-cell activity control[J]. Int J Cancer, 2023, 153(10): 1706-25. |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||