南方医科大学学报 ›› 2025, Vol. 45 ›› Issue (1): 43-51.doi: 10.12122/j.issn.1673-4254.2025.01.06
陈志亮1( ), 杨永刚2, 黄霞1, 成彦3, 瞿媛4, 衡琪琪5, 符羽佳5, 李可薇5, 顾宁1(
), 杨永刚2, 黄霞1, 成彦3, 瞿媛4, 衡琪琪5, 符羽佳5, 李可薇5, 顾宁1( )
)
收稿日期:2024-03-23
出版日期:2025-01-20
发布日期:2025-01-20
通讯作者:
顾宁
E-mail:czhilt@126.com;guning@njucm.edu.cn
作者简介:陈志亮,博士,副主任中医师,E-mail: czhilt@126.com
基金资助:
Zhiliang CHEN1( ), Yonggang YANG2, Xia HUANG1, Yan CHENG3, Yuan QU4, Qiqi HENG5, Yujia FU5, Kewei LI5, Ning GU1(
), Yonggang YANG2, Xia HUANG1, Yan CHENG3, Yuan QU4, Qiqi HENG5, Yujia FU5, Kewei LI5, Ning GU1( )
)
Received:2024-03-23
Online:2025-01-20
Published:2025-01-20
Contact:
Ning GU
E-mail:czhilt@126.com;guning@njucm.edu.cn
摘要:
目的 分析慢性心力衰竭(CHF)合并高尿酸血症(HUA)患者血清外泌体miRNA的差异表达,探讨其作为CHF合并HUA新型诊断分子标志物的可能性,并对差异miRNA进行靶基因功能分析,分析作用靶点。 方法 以2020年9月~2023年9月南京中医药大学附属南京中医院心血管病科收治的CHF合并HUA患者为观察组(n=30),选择同期健康志愿者为对照组(n=30)。两组各选取6例样本,采用高通量测序分析血清外泌体中的差异表达miRNA,采用RT-PCR检测对未作高通量测序的观察组和对照组样本(n=24)进行验证,使用R软件进行GO、KEGG富集分析,预测差异表达miRNA的作用靶点,并通过动物实验验证临床筛查的差异miRNA。 结果 高通量测序分析显示,观察组患者共检测到42个差异表达的miRNA(18个上调,24个下调),其中miR-27a-5p上调(P<0.001),miR-139-3p下调(P<0.001)。RT-PCR检测显示,观察组患者血清外泌体中miR-27a-5p表达量上调(P=0.004)、miR-139-3p表达量下调(P=0.005);ROC曲线下面积(AUC)分析发现,miR-27a-5p、miR-139-3p预测CHF合并HUA发病的AUC分别是0.708(95% CI:0.562-0.855)和0.734(95% CI:0.593-0.876),两者联合预测CHF与HUA发病的AUC为0.899(95% CI:0.812-0.987)。对差异基因进行GO富集分析发现,细胞自噬是富集程度最高的靶点;KEGG功能注释显示,激活AMPK-mTOR信号通路可能是差异表达的miR-27a-5p和miR-139-3p作用靶点之一。进一步动物实验得到了相同的验证。 结论 血清外泌体中miR-27a-5p上调和miR-139-3p下调可作为精准诊断CHF合并HUA的新型分子标志物,激活AMPK-mTOR信号通路后促进心肌细胞的自噬反应可能是差异表达的miR-27a-5p、miR-139-3p的作用靶点之一。
陈志亮, 杨永刚, 黄霞, 成彦, 瞿媛, 衡琪琪, 符羽佳, 李可薇, 顾宁. 外泌体miRNA差异表达可作为诊断慢性心力衰竭合并高尿酸血症患者新型分子标志物及靶基因功能分析[J]. 南方医科大学学报, 2025, 45(1): 43-51.
Zhiliang CHEN, Yonggang YANG, Xia HUANG, Yan CHENG, Yuan QU, Qiqi HENG, Yujia FU, Kewei LI, Ning GU. Differential expressions of exosomal miRNAs in patients with chronic heart failure and hyperuricemia: diagnostic values of miR-27a-5p and miR-139-3p[J]. Journal of Southern Medical University, 2025, 45(1): 43-51.
| Target genes | Primer | Sequences | Annealing temp (℃) | Product size (bp) | Notes |
|---|---|---|---|---|---|
| hsa-miR-27a-5p | hsa-miR-27a-5p-RT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTGCTCA | - | 66 | Stem ring primers |
| hsa-miR-27a-5p F | CCAGCGTGAGGGCTTAGC | 60 | Forward primers | ||
| microRNA_uniRev | CAGTGCAGGGTCCGAGGTAT | Reverse primers | |||
| hsa-miR-139-3p | hsa-miR-139-3p-RT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACTCCA | - | 67 | Stem ring primers |
| hsa-miR-139-3p F | CCAGCGTGTGGAGACGC | 60 | Forward primers | ||
| microRNA_uniRev | CAGTGCAGGGTCCGAGGTAT | Reverse primers | |||
| hsa-U6 | hsa-U6 snRNA For | CTCGCTTCGGCAGCACATA | 60 | 85 | Forward primers |
| hsa-U6 snRNA Rev | CGAATTTGCGTGTCATCCT | Reverse primers |
表1 引物信息
Tab.1 Primer sequences for qRT-PCR for miR-27a-5p, miR-139-3p and U6
| Target genes | Primer | Sequences | Annealing temp (℃) | Product size (bp) | Notes |
|---|---|---|---|---|---|
| hsa-miR-27a-5p | hsa-miR-27a-5p-RT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTGCTCA | - | 66 | Stem ring primers |
| hsa-miR-27a-5p F | CCAGCGTGAGGGCTTAGC | 60 | Forward primers | ||
| microRNA_uniRev | CAGTGCAGGGTCCGAGGTAT | Reverse primers | |||
| hsa-miR-139-3p | hsa-miR-139-3p-RT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACTCCA | - | 67 | Stem ring primers |
| hsa-miR-139-3p F | CCAGCGTGTGGAGACGC | 60 | Forward primers | ||
| microRNA_uniRev | CAGTGCAGGGTCCGAGGTAT | Reverse primers | |||
| hsa-U6 | hsa-U6 snRNA For | CTCGCTTCGGCAGCACATA | 60 | 85 | Forward primers |
| hsa-U6 snRNA Rev | CGAATTTGCGTGTCATCCT | Reverse primers |
| Characteristic | Observation group | Control group | P |
|---|---|---|---|
| Gender (%) | 0.417 | ||
| Male | 60 | 70 | |
| Female | 40 | 30 | |
| Age (year) | 67.03±18.10 | 63.07±13.08 | 0.335 |
| SUA (μmol/L) | 528.36±83.48 | 314.74±58.76 | <0.001 |
| LVEF (%) | 38.81±9.43 | 65.30±2.28 | <0.001 |
| ALT (U/L) | 30.80±23.04 | 25.73±13.66 | 0.305 |
| AST (U/L) | 28.20±16.53 | 21.20±9.95 | 0.051 |
| SCr (μmol/L) | 96.10±22.06 | 65.80±13.67 | <0.001 |
| BUN (mmol/L) | 7.19±1.79 | 5.27±1.30 | <0.001 |
| TC (mmol/L) | 4.44±1.20 | 4.94±0.90 | 0.067 |
表2 CHF合并HUA患者与健康志愿者一般资料比较
Tab.2 Baseline characteristics of patients with chronic heart failure (CHF) complicated by hyperuricemia (HUA) and healthy volunteers (Mean±SD, n=30)
| Characteristic | Observation group | Control group | P |
|---|---|---|---|
| Gender (%) | 0.417 | ||
| Male | 60 | 70 | |
| Female | 40 | 30 | |
| Age (year) | 67.03±18.10 | 63.07±13.08 | 0.335 |
| SUA (μmol/L) | 528.36±83.48 | 314.74±58.76 | <0.001 |
| LVEF (%) | 38.81±9.43 | 65.30±2.28 | <0.001 |
| ALT (U/L) | 30.80±23.04 | 25.73±13.66 | 0.305 |
| AST (U/L) | 28.20±16.53 | 21.20±9.95 | 0.051 |
| SCr (μmol/L) | 96.10±22.06 | 65.80±13.67 | <0.001 |
| BUN (mmol/L) | 7.19±1.79 | 5.27±1.30 | <0.001 |
| TC (mmol/L) | 4.44±1.20 | 4.94±0.90 | 0.067 |
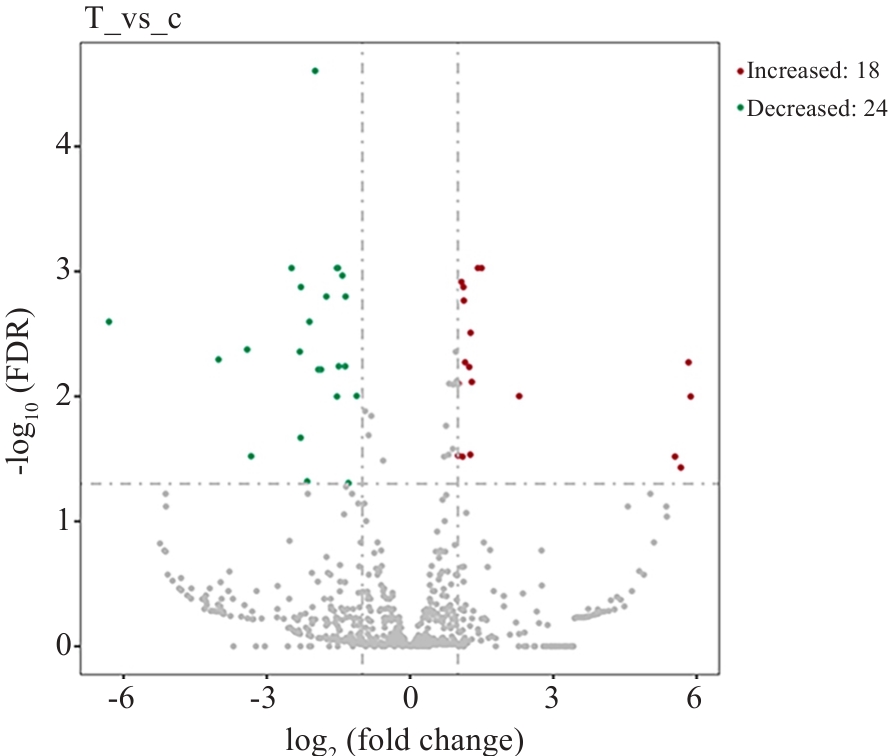
图1 差异miRNA火山图
Fig.1 Volcano plots of the differentially expressed exosomal miRNAs in CHF patients with HUA. Red and green dots represent the genes with significant differential expression, and gray dots represent the genes without substantial differential expression. Upregulated miRNAs are labeled in red, and downregulated ones are marked in green.
| Upregulated miRNAs | BaseMean | Log FC | P |
|---|---|---|---|
| MiR-27a-5p | 4.740923 | 5.8306 | 0.000179 |
| MiR-193b-3p | 64.58635 | 2.2848 | 0.000523 |
| MiR-210-5p | 4.917714 | 5.8752 | 0.000554 |
| MiR-508-5p | 4.249289 | 1.9014 | 0.002869 |
表3 CHF合并HUA患者部分表达上调的差异miRNA
Tab.3 Partial upregulated exosomal miRNAs in CHF patients with HUA versus healthy volunteers
| Upregulated miRNAs | BaseMean | Log FC | P |
|---|---|---|---|
| MiR-27a-5p | 4.740923 | 5.8306 | 0.000179 |
| MiR-193b-3p | 64.58635 | 2.2848 | 0.000523 |
| MiR-210-5p | 4.917714 | 5.8752 | 0.000554 |
| MiR-508-5p | 4.249289 | 1.9014 | 0.002869 |
| Downregulated miRNAs | BaseMean | Log FC | P |
|---|---|---|---|
| MiR-139-3p | 81.70276 | -2.1076 | <0.001 |
| MiR-4446-3p | 17.05699 | -3.4115 | <0.001 |
| MiR-654-3p | 104.6556 | -2.3092 | <0.001 |
| MiR-150-3p | 20.40789 | -2.1551 | <0.01 |
表4 CHF合并HUA患者部分表达下调的差异miRNA
Tab.4 Partial downregulated exosomal miRNAs in CHF patients with HUA versus healthy volunteers
| Downregulated miRNAs | BaseMean | Log FC | P |
|---|---|---|---|
| MiR-139-3p | 81.70276 | -2.1076 | <0.001 |
| MiR-4446-3p | 17.05699 | -3.4115 | <0.001 |
| MiR-654-3p | 104.6556 | -2.3092 | <0.001 |
| MiR-150-3p | 20.40789 | -2.1551 | <0.01 |
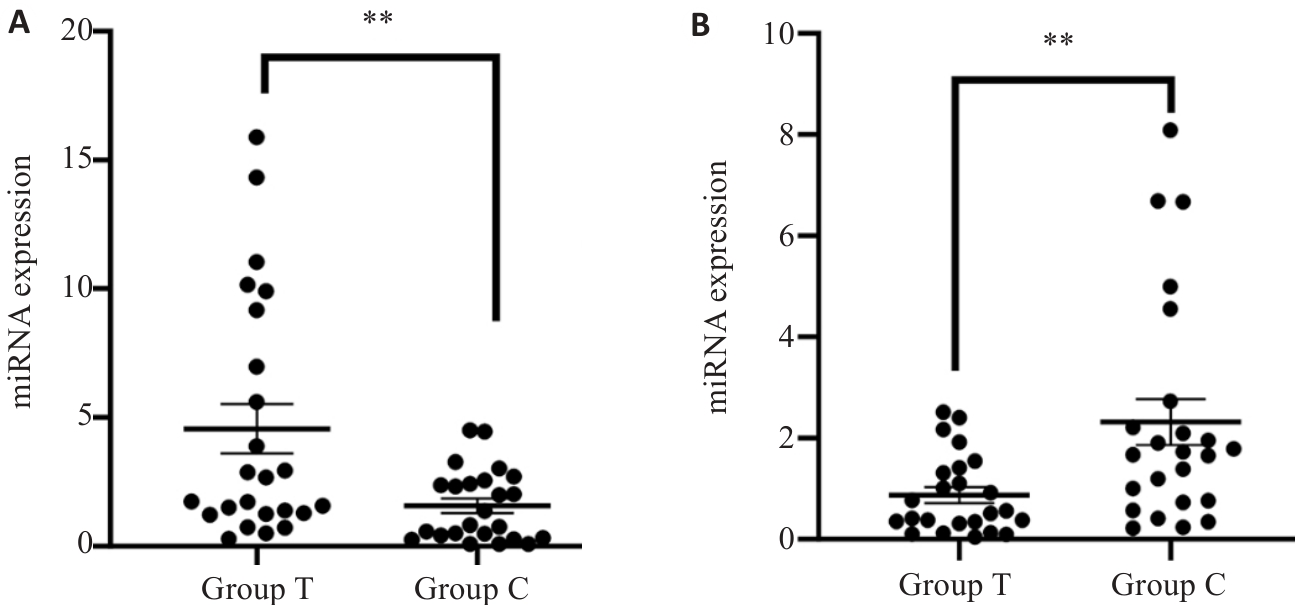
图2 血清外泌体miR-27a-5p(A)及miR-139-3p(B)表达情况
Fig.2 Relative levels of exosomal miR-27a-5p (A) and miR-139-3p (B) in CHF patients with HUA (group T) and healthy volunteers (group C). **P<0.01.
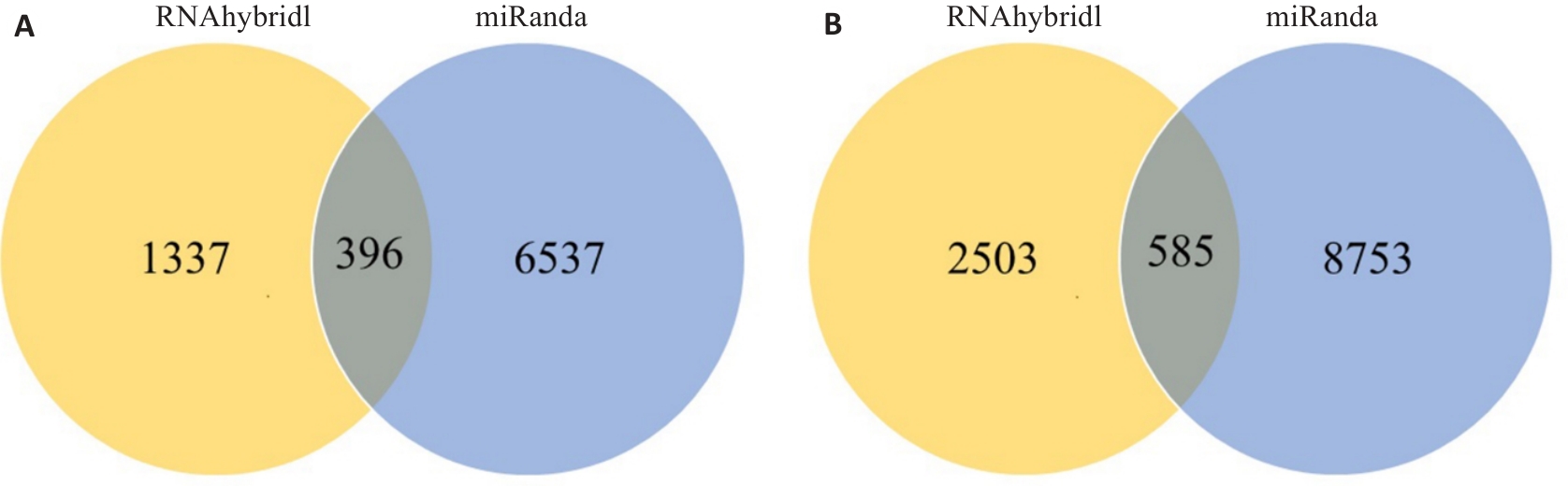
图3 miRanda、RNAhybridl软件预测miR-27a-5p (A)和miR-139-3p (B)靶基因的韦恩图
Fig.3 Venn diagram of the target genes of miR-27a-5p (A) and miR-139-3p (B) predicted by miRanda and RNAhybridl software.
| miRNAs | Target gene |
|---|---|
| MiR-27a-5p | ENST00000466134 |
| ENST00000474114 | |
| ENST00000262319 | |
| ENST00000415452 | |
| ENST00000395748 | |
| ENST00000502307 | |
| ENST00000511560 | |
| MiR-139-3p | ENST00000649865 |
| ENST00000531373 | |
| ENST00000650285 | |
| ENST00000261405 | |
| ENST00000262719 | |
| ENST00000358784 | |
| ENST00000480614 |
表5 筛选出的部分靶基因
Tab.5 Partial upregulated and downregulated exosomal miRNAs in CHF patients with HUA versus healthy volunteers
| miRNAs | Target gene |
|---|---|
| MiR-27a-5p | ENST00000466134 |
| ENST00000474114 | |
| ENST00000262319 | |
| ENST00000415452 | |
| ENST00000395748 | |
| ENST00000502307 | |
| ENST00000511560 | |
| MiR-139-3p | ENST00000649865 |
| ENST00000531373 | |
| ENST00000650285 | |
| ENST00000261405 | |
| ENST00000262719 | |
| ENST00000358784 | |
| ENST00000480614 |
| Diagnostic performance | miR-27a-5p | miR-139-3p | Combination detection |
|---|---|---|---|
| AUC (95% CI) | 0.708 (0.562-0.855) | 0.734 (0.593-0.876) | 0.899 (0.812-0.987) |
| P | 0.013 | 0.005 | 0.000 |
| Cut-off value | 0.652 | 1.603 | 0.483 |
| Sensitivity (%) | 91.7 | 83.3 | 79.2 |
| Specificity (%) | 41.7 | 58.3 | 91.7 |
| J value | 0.334 | 0.416 | 0.709 |
表6 血清外泌体miR-27a-5p、miR-139-3p 诊断CHF合并HUA的效能分析
Tab.6 Diagnostic performance of exosomal miR-27a-5p, exosomal miR-139-3p, and their combination for detection of CHF combined with HUA
| Diagnostic performance | miR-27a-5p | miR-139-3p | Combination detection |
|---|---|---|---|
| AUC (95% CI) | 0.708 (0.562-0.855) | 0.734 (0.593-0.876) | 0.899 (0.812-0.987) |
| P | 0.013 | 0.005 | 0.000 |
| Cut-off value | 0.652 | 1.603 | 0.483 |
| Sensitivity (%) | 91.7 | 83.3 | 79.2 |
| Specificity (%) | 41.7 | 58.3 | 91.7 |
| J value | 0.334 | 0.416 | 0.709 |
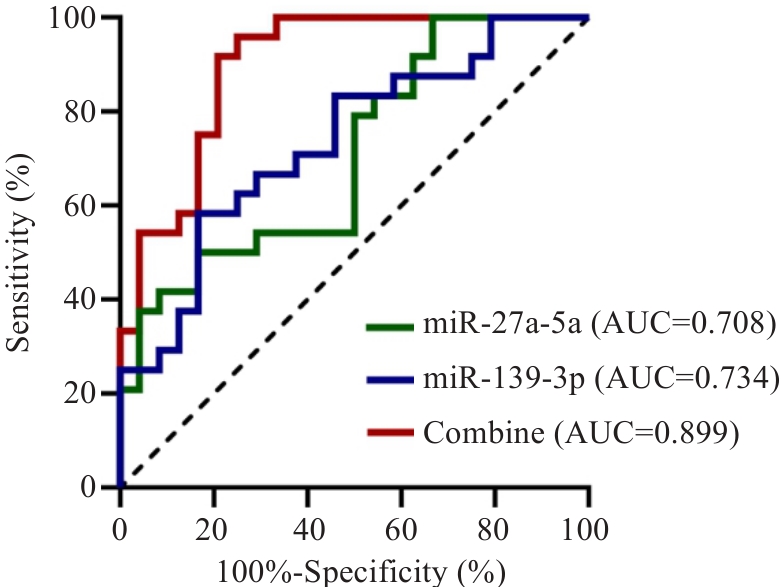
图4 miR-27a-5p、miR-139-3p预测CHF合并HUA的ROC曲线
Fig.4 ROC curves of the diagnostic potentials of exosomal miR-27a-5p and exosomal miR-139-3p for CHF combined with HUA.
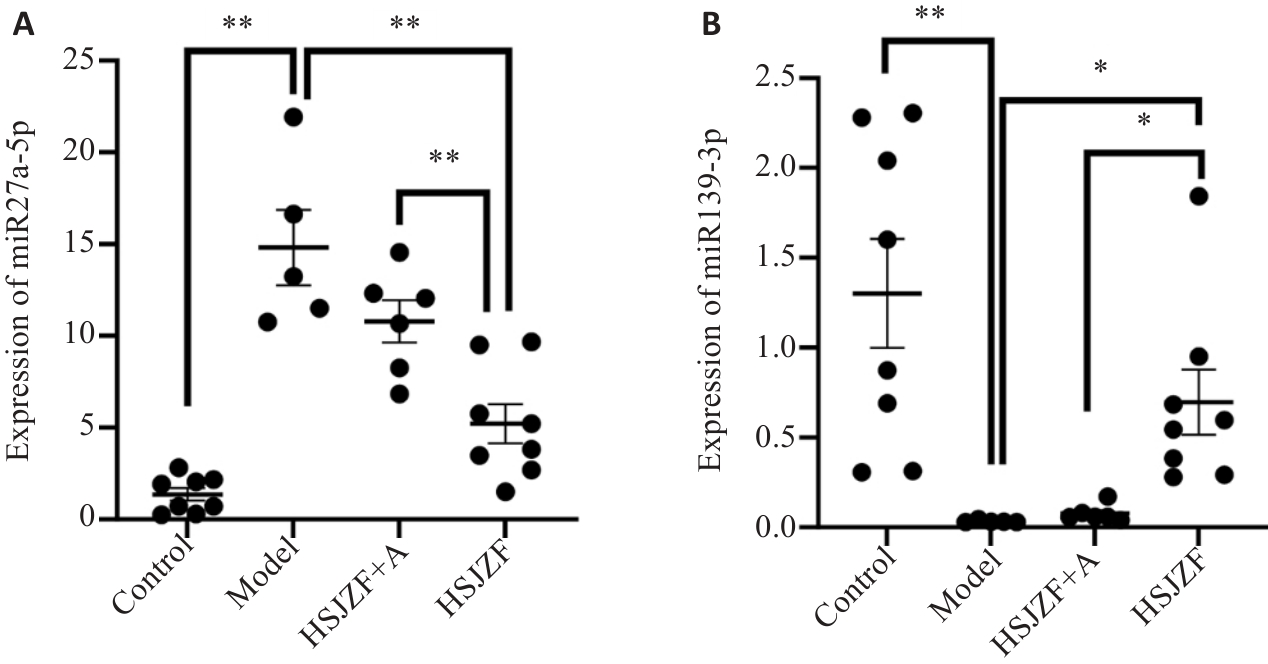
图6 大鼠干预后miR-27a-5p(A)及miR-139-3p(B)相对表达量比较
Fig.6 Comparison of relative expression of miR-27a-5p (A) and miR-139-3p (B) in 4 groups of rats after intervention. *P<0.05, **P<0.01.
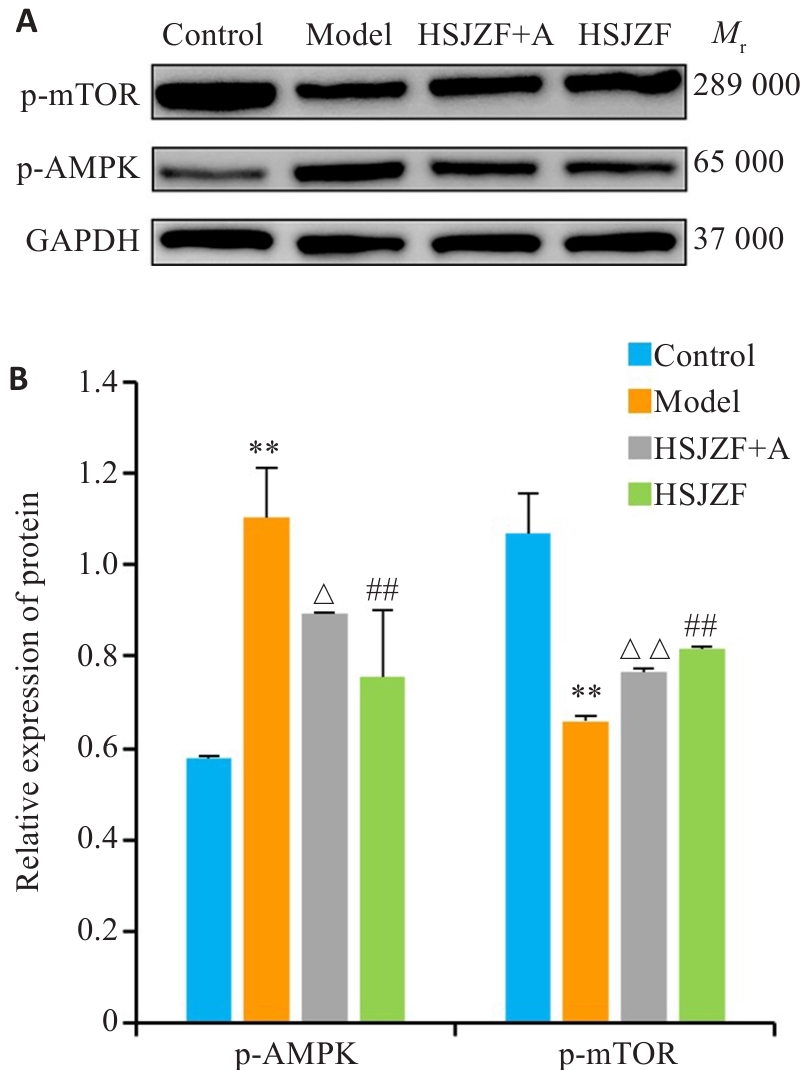
图7 大鼠心肌组织中p-AMPK和p-mTOR蛋白水平表达
Fig.7 Expression of p-AMPK and p-mTOR proteins in rat myocardial tissue in the 4 groups. **P<0.01 vs Control; ##P<0.01 vs Model; △P<0.05 vs HSJZF, △△P<0.01 vs HSJZF.
| 1 | Hamaguchi S, Furumoto T, Tsuchihashi-Makaya M, et al. Hyperuricemia predicts adverse outcomes in patients with heart failure[J]. Int J Cardiol, 2011, 151(2): 143-7. |
| 2 | Krishnan E. Hyperuricemia and incident heart failure[J]. Circ Heart Fail, 2009, 2(6): 556-62. |
| 3 | Huang H, Huang BT, Li YL, et al. Uric acid and risk of heart failure: a systematic review and meta-analysis[J]. Eur J Heart Fail, 2014, 16(1): 15-24. |
| 4 | 王晓瑜. 慢性肾脏病非透析患者BNP的影响因素及其与心功能关系的研究[D]. 广州: 广州医科大学, 2020: 33-6. |
| 5 | Takase H, Dohi Y. Kidney function crucially affects B-type natriuretic peptide (BNP), N-terminal proBNP and their relationship[J]. Eur J Clin Invest, 2014, 44(3): 303-8. |
| 6 | Vickery S, Price CP, John RI, et al. B-type natriuretic peptide (BNP) and amino-terminal proBNP in patients with CKD: relationship to renal function and left ventricular hypertrophy[J]. Am J Kidney Dis, 2005, 46(4): 610-20. |
| 7 | Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American college of cardiology/american heart association joint committee on clinical practice guidelines[J]. Circulation, 2022, 145(18): e895-1032. |
| 8 | Dong XK, Zhang HL, Wang F, et al. Epidemiology and prevalence of hyperuricemia among men and women in Chinese rural population: the Henan Rural Cohort Study[J]. Mod Rheumatol, 2020, 30(5): 910-20. |
| 9 | Saheera S, Potnuri AG, Krishnamurthy P. Nano-vesicle (mis)communication in senescence-related pathologies[J]. Cells, 2020, 9(9): 1974. |
| 10 | Zhang TM, Ma SH, Lv JK, et al. The emerging role of exosomes in Alzheimer's disease[J]. Ageing Res Rev, 2021, 68: 101321. |
| 11 | Guo M, Hao YN, Feng YW, et al. Microglial exosomes in neurodegenerative disease[J]. Front Mol Neurosci, 2021, 14: 630808. |
| 12 | Jiang H, Wang JX, Li M, et al. miRTRS: a recommendation algorithm for predicting miRNA targets[J]. IEEE/ACM Trans Comput Biol Bioinform, 2020, 17(3): 1032-41. |
| 13 | Vishnoi A, Rani S. MiRNA biogenesis and regulation of diseases: an overview[J]. Methods Mol Biol, 2017, 1509: 1-10. |
| 14 | Bheri S, Kassouf BP, Park HJ, et al. Engineering cardiac small extracellular vesicle-derived vehicles with thin-film hydration for customized microRNA loading[J]. J Cardiovasc Dev Dis, 2021, 8(11): 135. |
| 15 | Ameres SL, Zamore PD. Diversifying microRNA sequence and function[J]. Nat Rev Mol Cell Biol, 2013, 14(8): 475-88. |
| 16 | Chen H, Xue RC, Huang PS, et al. Modified exosomes: a good transporter for miRNAs within stem cells to treat ischemic heart disease[J]. J Cardiovasc Transl Res, 2022, 15(3): 514-23. |
| 17 | Wang L, Liu J, Xu B, et al. Reduced exosome miR-425 and miR-744 in the plasma represents the progression of fibrosis and heart failure[J]. Kaohsiung J Med Sci, 2018, 34(11): 626-33. |
| 18 | Gou HM, Wan PC, Guo WQ, et al. Analysis of miRNA expression profile of hyperuricemia in peripheral blood and the construction of ceRNA network [J]. J Nor Sichuan Med Coll, 2023, 38(04): 451-6. |
| 19 | 高 娜. 应用超声生物显微镜评价肥胖小鼠心肌功能与其外泌体成分的实验研究[D]. 银川: 宁夏医科大学, 2022: 25-7. |
| 20 | Ning Y, Huang PS, Chen GH, et al. Atorvastatin-pretreated mesenchymal stem cell-derived extracellular vesicles promote cardiac repair after myocardial infarction via shifting macrophage polarization by targeting microRNA-139-3p/Stat1 pathway[J]. BMC Med, 2023, 21(1): 96. |
| 21 | Zhang Q, Chen L, Huang LY, et al. CD44 promotes angiogenesis in myocardial infarction through regulating plasma exosome uptake and further enhancing FGFR2 signaling transduction[J]. Mol Med, 2022, 28(1): 145. |
| 22 | Wang XJ, Morelli MB, Matarese A, et al. Cardiomyocyte-derived exosomal microRNA-92a mediates post-ischemic myofibroblast activation both in vitro and ex vivo [J]. ESC Heart Fail, 2020, 7(1): 284-8. |
| 23 | Morelli MB, Shu J, Sardu C, et al. Cardiosomal microRNAs are essential in post-infarction myofibroblast phenoconversion[J]. Int J Mol Sci, 2019, 21(1): 201. |
| 24 | Kansakar U, Varzideh F, Mone P, et al. Functional role of microRNAs in regulating cardiomyocyte death[J]. Cells, 2022, 11(6): 983. |
| 25 | Zhang DQ, Zheng N, Fu XL, et al. Dl-3-n-butylphthalide attenuates myocardial ischemia reperfusion injury by suppressing oxidative stress and regulating cardiac mitophagy via the PINK1/Parkin pathway in rats[J]. J Thorac Dis, 2022, 14(5): 1651-62. |
| 26 | Li B, Chi RF, Qin FZ, et al. Distinct changes of myocyte autophagy during myocardial hypertrophy and heart failure: association with oxidative stress[J]. Exp Physiol, 2016, 101(8): 1050-63. |
| 27 | Wang JL, Li YZ, Tao TQ, et al. Postconditioning with calreticulin attenuates myocardial ischemia/reperfusion injury and improves autophagic flux[J]. Shock, 2020, 53(3): 363-72. |
| 28 | Chen YC, Zhang CL, Wu Q. The role of autophagy in vascular endothelial cell damage and inflammatory response caused by high uric acid [J]. Chin J Gerontol, 2019, 39(24): 6098-101. |
| 29 | Du LG, Yu Y, Lan QS, et al. Lesinurad improves high uric acid-induced abnormal proliferation of vascular smooth muscle by inhibiting autophagy via gegulating AMPK/mTOR pathway [J]. J Guangzhou Med Univ, 2022, 50(5): 1-5. |
| 30 | Liu D, Gao K, Xie Y, et al. The effect of high uric acid on the activity of cardiomyocytes and its related mechanism [J]. Tianjin Med J, 2020, 48(10): 931-6. |
| 31 | Li TY, Lin SY, Lin SC. Mechanism and physiological significance of growth factor-related autophagy[J]. Physiology, 2013, 28(6): 423-31. |
| 32 | Han DD, Jiang LL, Gu XL, et al. SIRT3 deficiency is resistant to autophagy-dependent ferroptosis by inhibiting the AMPK/mTOR pathway and promoting GPX4 levels[J]. J Cell Physiol, 2020, 235(11): 8839-51. |
| 33 | Li WM, Zhu JQ, Dou J, et al. Phosphorylation of LAMP2A by p38 MAPK couples ER stress to chaperone-mediated autophagy[J]. Nat Commun, 2017, 8(1): 1763. |
| 34 | Lou DX, Zhang XG, Jiang CH, et al. 3β, 23-dihydroxy-12-ene-28-ursolic acid isolated from Cyclocarya paliurus alleviates NLRP3 inflammasome-mediated gout via PI3K-AKT-mTOR-dependent autophagy[J]. Evid Based Complement Alternat Med, 2022, 2022: 5541232. |
| 35 | Ba LN, Gao JQ, Chen YP, et al. Allicin attenuates pathological cardiac hypertrophy by inhibiting autophagy via activation of PI3K/Akt/mTOR and MAPK/ERK/mTOR signaling pathways[J]. Phytomedicine, 2019, 58: 152765. |
| 36 | Fan CL, Cai WJ, Ye MN, et al. Qili Qiangxin, a compound herbal medicine formula, alleviates hypoxia-reoxygenation-induced apoptotic and autophagic cell death via suppression of ROS/AMPK/mTOR pathway in vitro [J]. J Integr Med, 2022, 20(4): 365-75. |
| [1] | 李玮怡, 江露, 张宗星, 陈丹, 包卓玛, 黄丽, 袁林. 强骨康疏方通过抑制HIF-1α/BNIP3自噬信号通路减少类风湿性关节炎大鼠的破骨细胞分化[J]. 南方医科大学学报, 2025, 45(7): 1389-1396. |
| [2] | 吴璇, 方家敏, 韩玮玮, 陈琳, 孙菁, 金齐力. 高表达PRELID1促进胃癌细胞上皮间质转化并与不良预后相关[J]. 南方医科大学学报, 2025, 45(7): 1535-1542. |
| [3] | 王心恒, 邵小涵, 李童童, 张璐, 杨勤军, 叶卫东, 童佳兵, 李泽庚, 方向明. 平喘宁方通过调控HMGB1/Beclin-1轴介导的自噬改善患寒哮证大鼠的气道炎症[J]. 南方医科大学学报, 2025, 45(6): 1153-1162. |
| [4] | 涂舒谕, 陈祥宇, 李程辉, 黄丹萍, 张莉. 补阳还五汤通过调控外泌体miR-590-5p介导的巨噬细胞极化延缓大鼠血管衰老[J]. 南方医科大学学报, 2025, 45(6): 1251-1259. |
| [5] | 张璐, 丁焕章, 许浩燃, 陈珂, 许博文, 杨勤军, 吴迪, 童佳兵, 李泽庚. 参芪补中方通过激活AMPK/SIRT1/PGC-1α改善COPD肺脾气虚证大鼠线粒体功能障碍[J]. 南方医科大学学报, 2025, 45(5): 969-976. |
| [6] | 董妍妍, 张可敬, 储俊, 储全根. 抵当汤含药血清通过PI3K/Akt/mTOR信号通路增强高糖诱导的大鼠肾小球内皮细胞自噬[J]. 南方医科大学学报, 2025, 45(3): 461-469. |
| [7] | 廖茗, 钟文华, 张冉, 梁娟, 徐文陶睿, 万文珺, 吴超, 李曙. 源自蛇毒的蛋白C激活剂通过调控HIF-1α抑制BNIP3活性氧生成保护人脐静脉内皮细胞免受缺氧-复氧损伤[J]. 南方医科大学学报, 2025, 45(3): 614-621. |
| [8] | 石情, 冉苏叶, 宋铃榆, 杨红, 王文娟, 刘晗琳, 刘琦. NLRP6过表达通过AMPK/CPT1A/PGC1A通路促进肝细胞脂肪氧化分解改善非酒精性脂肪肝[J]. 南方医科大学学报, 2025, 45(1): 118-125. |
| [9] | 郭克磊, 李颖利, 宣晨光, 侯紫君, 叶松山, 李林运, 陈丽平, 韩立, 卞华. 益气养阴化浊通络方通过调控miR-21a-5p/FoxO1/PINK1介导的线粒体自噬减轻糖尿病肾病小鼠的足细胞损伤[J]. 南方医科大学学报, 2025, 45(1): 27-34. |
| [10] | 展俊平, 黄硕, 孟庆良, 范围, 谷慧敏, 崔家康, 王慧莲. 缺氧微环境下补阳还五汤通过抑制BNIP3-PI3K/Akt通路抑制类风湿关节炎滑膜成纤维细胞的线粒体自噬[J]. 南方医科大学学报, 2025, 45(1): 35-42. |
| [11] | 何思齐, 文楠, 陈勋, 王跃, 张艇, 牟雁东. 枸杞糖肽可减轻放射治疗后人牙龈成纤维细胞来源的外泌体导致的成骨抑制[J]. 南方医科大学学报, 2024, 44(9): 1752-1759. |
| [12] | 戴荣, 曹泽平, 刘传娇, 葛永, 程梦, 王伟丽, 陈义珍, 张磊, 王亿平. 清肾颗粒通过调控外泌体、miR-330-3p以及CREBBP表达抑制小鼠肾纤维化[J]. 南方医科大学学报, 2024, 44(8): 1431-1440. |
| [13] | 耿志军, 杨晶晶, 牛民主, 刘馨悦, 施金冉, 刘亦珂, 姚新宇, 张雨路, 张小凤, 胡建国. 桑黄酮G通过调控PI3K/AKT/mTOR通路抑制胃癌细胞的生长、迁移和侵袭[J]. 南方医科大学学报, 2024, 44(8): 1476-1484. |
| [14] | 程瑶, 王远迎, 姚飞扬, 胡盼, 陈铭勰, 吴宁. 黄芩苷通过调控PI3K/AKT信号通路抑制登革病毒感染诱导的人静脉内皮细胞的自噬[J]. 南方医科大学学报, 2024, 44(7): 1272-1283. |
| [15] | 张叶明, 张袁祥, 沈学彬, 王国栋, 朱磊. 在抑郁症大鼠模型中MiRNA-103-3p调控Rab10促进神经细胞自噬[J]. 南方医科大学学报, 2024, 44(7): 1315-1326. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||